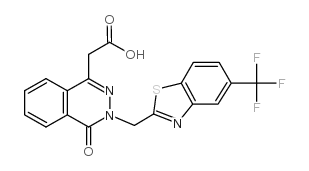
110703-94-1
| Name | Zopolrestat |
|---|---|
| Synonyms | 2-[4-oxo-3-[[5-(trifluoromethyl)-1,3-benzothiazol-2-yl]methyl]phthalazin-1-yl]acetic acid |
| Description | Zopolrestat (CP73850) is a potent, orally active aldose reductase (AR) inhibitor with an IC50 of 3.1 nM. Zopolrestat is used for the research of diabetic complications[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Zopolrestat is a potent inhibitor of the reduction of both glyceraldehyde and glucose by the human and rat enzymes[1]. |
| In Vivo | Zopolrestat (2.5 mg/kg-50 mg/kg; p.o.; once-a-day for 5 days) and left untreated for 7 days) prevents accumulation of sorbitol in the kidney cortex of diabetic rats and normalize elevated renal blood flow in galactosemic rats[1]. Animal Model: Male Sprague-Dawley rats (made diabetic by iv injection of streptozotocin)[1]. Dosage: 2.5 mg/kg-50 mg/kg Administration: P.o.; once-a-day for 5 days Result: Its ED50s in reversing already elevated sorbitol accumulation in rat sciatic nerve, retina, and lens in a chronic test were 1.9, 17.6, and 18.4 mg/kg, respectively. |
| References |
| Density | 1.58g/cm3 |
|---|---|
| Boiling Point | 598.7ºC at 760mmHg |
| Molecular Formula | C19H12F3N3O3S |
| Molecular Weight | 419.37700 |
| Flash Point | 315.9ºC |
| Exact Mass | 419.05500 |
| PSA | 113.32000 |
| LogP | 3.70040 |
| Vapour Pressure | 3.5E-15mmHg at 25°C |
| Index of Refraction | 1.683 |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H413 |
| Precautionary Statements | P301 + P310 |
| Hazard Codes | T+ |
| RIDADR | UN 2811 6.1 / PGIII |