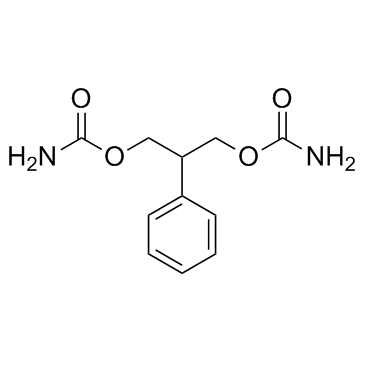
25451-15-4
| Name | felbamate |
|---|---|
| Synonyms |
Felbatol
3-propanediol dicarbamate EINECS 247-001-4 Felbamatum Felbamato 1,3-Propanediol,2-phenyl-,dicarbamate 1,3-bis-carbamoyloxy-2-phenyl-propane 1,3-Bis-carbamoyloxy-2-phenyl-propan MFCD00865296 2-Phenyl-1,3-propanediyl dicarbamate felbamate Felbamyl (3-carbamoyloxy-2-phenylpropyl) carbamate Carbamic acid,2-phenyltrimethylene ester 1,3-Propanediol, 2-phenyl-, dicarbamate Taloxa 2-Phenylpropane-1,3-diyl dicarbamate 2-phenyl-1,3-propanediol dicarbamate |
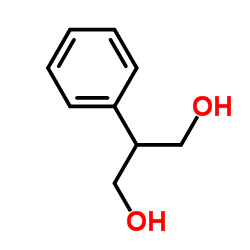
| Description | Felbamate (FBM) is a potent nonsedative anticonvulsant whose clinical effect may be related to the inhibition of N-methyl-D-aspartate (NMDA) .Target: NMDA ReceptorFelbamate (marketed under the brand name Felbatol by MedPointe) is an anti-epileptic drug used in the treatment of epilepsy. It is used to treat partial seizures (with and without generalization) in adults and partial and generalized seizures associated with Lennox-Gastaut syndrome in children. However, an increased risk of potentially fatal aplastic anemia and/or liver failure limit the drugs usage to severe refractory epilepsy.Felbamate has been proposed to a unique dual mechanism of action as a positive modulator of GABAA receptors and as a blocker of NMDA receptors, particularly isoforms containing the NR2B subunit. Although it is clear that felbamate does cause pharmacological inhibition of NMDA receptor of relevance of NMDA receptor blockade as a strategy for the treatment of human epilepsy has been questioned. Therefore, the importance of the effects of felbamate on NMDA receptors to its therapeutic action in epilepsy is uncertain. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 511.9±50.0 °C at 760 mmHg |
| Melting Point | 148-1500C |
| Molecular Formula | C11H14N2O4 |
| Molecular Weight | 238.240 |
| Flash Point | 288.4±26.4 °C |
| Exact Mass | 238.095352 |
| PSA | 104.64000 |
| LogP | 1.20 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.559 |
| Storage condition | Store at RT |
| Water Solubility | alcohol: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol |
| WGK Germany | 2 |
| RTECS | TZ1070000 |
| HS Code | 2924299090 |
|
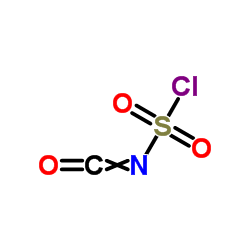
~% 
25451-15-4 |
| Literature: WO2012/32508 A1, ; Page/Page column 6 ; |
|
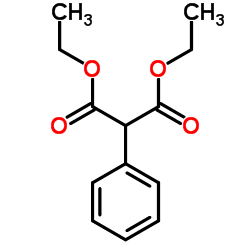
~80% 
25451-15-4 |
| Literature: Macdonald, Timothy L. Patent: US2002/156070 A1, 2002 ; |
|
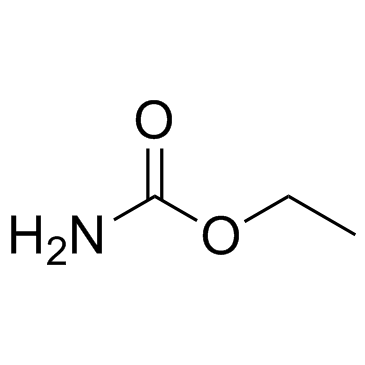
~% 
25451-15-4 |
| Literature: Chemical Research in Toxicology, , vol. 10, # 4 p. 457 - 462 |
|
~% 
25451-15-4 |
| Literature: US2009/111871 A1, ; Page/Page column 3 ; |
|
~% 
25451-15-4 |
| Literature: Chemical Research in Toxicology, , vol. 10, # 4 p. 457 - 462 |
|
~% 
25451-15-4 |
| Literature: WO2012/32508 A1, ; |
|
~% 
25451-15-4 |
| Literature: US2884444 , ; |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |