61574-54-7
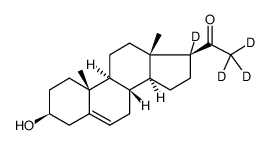
| Name | pregnenolone-17alpha,21,21,21-d4 |
|---|---|
| Synonyms |
1,2:4,5-di-O-cyclohexylidene inositol
1,2:4,5-Biscyclohexylidene DL-myo-Inositol 1,2:4,5-Di-O-cyclohexylidene-D,L-myo-inositol 1,2:4,5-dicyclohexylidene-myo-inositol 1,2:4,5-di-O,O-cyclohexylidene-myo-inositol 17,21,21,21-d4-pregnenolone |
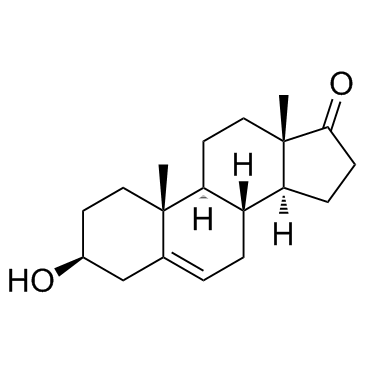
| Description | Pregnenolone-d4-1 (3β-Hydroxy-5-pregnen-20-one-d4-1) is the deuterium labeled Pregnenolone. Pregnenolone (3β-Hydroxy-5-pregnen-20-one) is a powerful neurosteroid, the main precursor of various steroid hormones including steroid ketones. Pregnenolone acts as a signaling-specific inhibitor of cannabinoid CB1 receptor, inhibits the effects of tetrahydrocannabinol (THC) that are mediated by the CB1 receptors. Pregnenolone can protect the brain from cannabis intoxication[1][2]. Pregnenolone is also a TRPM3 channel activator, and also can weakly activate TRPM1 channels[3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C21H28D4O2 |
|---|---|
| Molecular Weight | 320.50200 |
| Exact Mass | 320.26500 |
| PSA | 37.30000 |
| LogP | 4.51530 |