1282512-48-4
| Name | Taselisib |
|---|---|
| Synonyms |
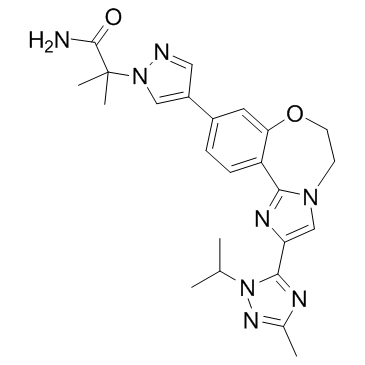
2-{4-[2-(1-Isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydroimidazo[1,2-d][1,4]benzoxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide
GDC-0032 RG7604 1H-Pyrazole-1-acetamide, 4-[5,6-dihydro-2-[3-methyl-1-(1-methylethyl)-1H-1,2,4-triazol-5-yl]imidazo[1,2-d][1,4]benzoxazepin-9-yl]-α,α-dimethyl- S7103,GDC0032,RG7604 UNII-L08J2O299M 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f ]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanamide 2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl)-1H-pyrazol-1-yl)-2-methylpropanoic acid Taselisib |
| Description | Taselisib (GDC-0032) is a potent β-sparing small molecule inhibitor of PI3K, with Ki values of 0.29 nM, 0.91 nM, 0.97 nM for PI3Kα, PI3Kβ and PI3Kγ, respectively. |
|---|---|
| Related Catalog | |
| Target |
PI3Kα:0.29 nM (Ki) PI3Kβ:9.1 nM (Ki) PI3Kδ:0.12 nM (Ki) PI3Kγ:0.97 nM (Ki) |
| In Vitro | Taselisib (GDC-0032) (100 nM) inhibits AKT/mTOR signaling in PIK3CA mutant cell lines but not in cells with loss or mutation of PTEN; Taselisib (GDC-0032) enhances radiation-induced apoptosis and inhibits growth in head and neck cancer cell lines that are sensitive to its single-agent activiy[1]. Taselisib (GDC-0032) enhances the effects of MEK1/2 inhibition on both BRAFV600E/PTENNull human melanoma cells autochthonous mouse melanomas[2]. |
| In Vivo | Taselisib (GDC-0032) (5 mg/kg, p.o.) potently impairs PI3K signaling and enhances the efficacy of fractionated radiotherapy; Taselisib (GDC-0032) and radiation is more effective than either treatment alone in nude mice implanted with subcutaneous Cal-33 xenografts[1]. The vehicle-treated BRAFV600E/PTENNull melanoma-bearing mice experiencs initial tumor regression after treatment with Taselisib (GDC-0032) (22.5 mg/kg, p.o.)[2]. |
| Cell Assay | Cells are seeded in replicates of 6 in 96-well plates with 500 to 5,000 cells/well overnight and then treated with Taselisib (GDC-0032). After 4 days, the media are removed and the cells are fixed with 4% glutaraldehyde for 30 minutes. Fixed cells are stained with 0.1% crystal violet for 2 minutes, then washed, and dissolved in 10% acetic acid. |
| Animal Admin | Six-week-old Nu/Nu mice are injected bilaterally with 5×105 cells resuspended in 200 μL of culture media and Matrigel mixed in a 1:1 ratio. After tumors reache approximately 100 to 200 cm3, mice are randomized into treatment arms with 8 to 10 tumors per group. Taselisib (GDC-0032) (5 mg/kg) is dissolved in a vehicle containing 0.5% methylcellulose with 0.2% TWEEN-80 and is administered via daily oral gavage. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 783.3±70.0 °C at 760 mmHg |
| Molecular Formula | C24H28N8O2 |
| Molecular Weight | 460.531 |
| Flash Point | 427.5±35.7 °C |
| Exact Mass | 460.233521 |
| PSA | 119.66000 |
| LogP | 0.69 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.709 |
| Storage condition | -20℃ |
| Precursor 6 | |
|---|---|
| DownStream 0 | |