11000-17-2
| Name | Vasopressin |
|---|---|
| Synonyms |
pituitrin
EINECS 200-050-5 VASOPRESSIN ACETATE MFCD03839092 vasophysin leiormone tonephin VASOPRASSIN adh(hormone) pitressin |
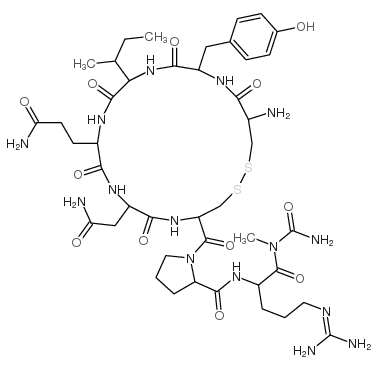
| Description | Vasopressin is a cyclic nonapeptide that is synthesized centrally in the hypothalamus. Vasopressin participates in the hypothalamic-pituitary-adrenal axis, and regulates pituitary corticotropin secretion by potentiating the stimulatory effects of corticotropin releasing factor. Vasopressin also can act as a neurotransmitter, exerting its action by binding to specific G protein-coupled receptors[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | AVP (0.01 nM-1 μM) induces Ca2+ increase in Chinese hamster ovary cells expressing rat or human V1b receptors[2]. |
| In Vivo | Vasopressin (0.03-0.3 μg/kg; i.p.) potentiates corticotropin release provoked by exogenous corticoliberin and increases corticotropin secretion subsequent to body water loss[2]. Vasopressin (0.001-0.1 mg/kg; i.p.) potently increases adjacent lying, where rats meeting for the first time lie passively next to each other[3]. |
| References |
| Molecular Formula | C43H67N15O12S2 |
|---|---|
| Molecular Weight | 1050.22000 |
| Exact Mass | 1049.45000 |
| PSA | 505.74000 |
| LogP | 1.50910 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|