848695-25-0
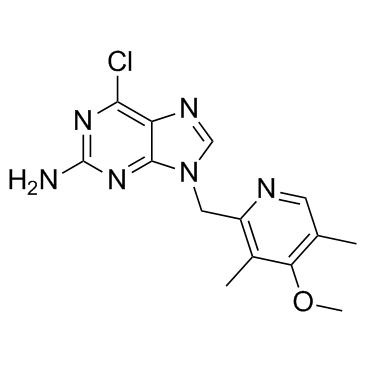
| Name | 6-chloro-9-[(4-methoxy-3,5-dimethylpyridin-2-yl)methyl]purin-2-amine |
|---|---|
| Synonyms |
6-Chloro-9-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]-9H-purin-2-amine
cnf2024 9H-Purin-2-amine, 6-chloro-9-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]- unii-851b9fq7q0 biib021 6-chloro-9-(4-methoxy-3,5-dimethylpyridin-2-ylmethyl)-9H-purin-2-ylamine |
| Description | BIIB021 is an orally available, fully synthetic inhibitor of HSP90 with Ki and EC50 of 1.7 nM and 38 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
HSP90:1.7 nM (Ki) |
| In Vitro | BIIB021 binds in the ATP-binding pocket of Hsp90, interferes with Hsp90 chaperone function, and results in client protein degradation and tumor growth inhibition. BIIB021 inhibits tumor cell (BT474, MCF-7, N87, HT29, H1650, H1299, H69 and H82) proliferation with IC50 from 0.06-0.31 μM. BIIB021 induces the degradation of Hsp90 client proteins including HER-2, Akt, and Raf-1 and up-regulated expression of the heat shock proteins Hsp70 and Hsp27[1]. BIIB021 inhibits Hodgkin's lymphoma cells (KM-H2, L428, L540, L540cy, L591, L1236 and DEV) with IC50 from 0.24-0.8 μM. BIIB021 shows low activity in lymphocytes from healthy individuals. BIIB021 inhibits the constitutive activity of NF-κB despite defective IκB. BIIB021 induces the expression of ligands for the activating NK cell receptor NKG2D on Hodgkin's lymphoma cells resulting in an increased susceptibility to NK cell-mediated killing[2]. BIIB021 enhances the in vitro radiosensitivity of HNSCCA cell lines (UM11B and JHU12) with a corresponding reduction in the expression of key radioresponsive proteins, increases apoptotic cells and enhances G2 arrest[3]. BIIB021 is considerably more active than 17-AAG against adrenocortical carcinoma H295R. The cytotoxic activity of BIIB021 is not influenced by loss of NQO1 or Bcl-2 overexpression, molecular lesions that do not prevent client loss but are nonetheless associated with reduced cell killing by 17-AAG. BIIB021 is also active in 17-AAG resistant cell lines (NIH-H69, MES SA Dx5, NCI-ADR-RES, Nalm6)[4]. |
| In Vivo | Oral administration of BIIB021 leads to tumor growth inhibition in many tumor xenograft models including N87, BT474, CWR22, U87, SKOV3 and Panc-1[1]. BIIB021 effectively inhibits growth of L540cy tumor at a dose of 120 mg/kg[2]. BIIB021 significantly enhances antitumor growth effect of radiation in JHU12 xenograft[3]. |
| Kinase Assay | For fluorescence polarization competition measurements, the FITC-geldanamycin probe (20 nM) is reduced with 2 mM TCEP at room temperature for 3 hours, after which the solution is aliquoted and stored at -80°C until used. Recombinant human Hsp90α (0.8 nM) and reduced FITC-geldanamycin (2 nM) are incubated in a 96-well microplate at room temperature for 3 hours in the presence of assay buffer containing 20 mM HEPES (pH 7.4), 50 mM KCl, 5 mM MgCl2, 20 mM Na2MoO4, 2 mM DTT, 0.1 mg/mL BGG, and 0.1% (v/v) CHAPS. Following this preincubation, BIIB021 in 100% DMSO is then added to final concentrations of 0.2 nM to 10 μM (final volume 100 μL, 2% DMSO). The reaction is incubated for 16 hours at room temperature and fluorescence is then measured in an Analyst plate reader, excitation=485 nm, emission=535 nm. High and low controls contain no BIIB021 or no Hsp90, respectively. The data are fit to a four-parameter curve and IC50 is generated. |
| Cell Assay | A modified tetrazolium salt assay is used to measure the IC50. Tumor cells are added to 96-well plates and propagated for 24 hours before BIIB021 addition. BIIB021 is added to the plated cells. DMSO (0.03-0.003%) is included as a vehicle control. After incubation phenazine methosulfate (stock concentration 1 mg/mL) and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (stock concentration 2 mg/mL) are mixed at a ratio of 1:20 and added to each well of a 96-well plate. Reduction of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt gives rise to a soluble formazan product that is secreted into the culture medium. After 4 hours incubation, the formazan product is quantitated spectrophotometrically at a wavelength of 490 nm. Data are acquired using SOFTmaxPRO software, and 100% viability is defined as the A490 of DMSO-treated cells stained with 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (the mean A490 of cells treated with DMSO at a range of 0.03-0.003%). Percent viability of each sample is calculated from the A490 values as follows: % viability=(A490 nm sample/A490 nm DMSO-treated cells × 100). The IC50 is defined as the concentration that gives rise to 50% inhibition of cell viability. |
| Animal Admin | BALB/c and athymic mice are obtained from Harlan Sprague-Dawley at age 6 to 8 weeks. The mice are maintained in sterilized cages in a ventilated caging system with a 12 h light/12 h dark photoperiod at temperature of 21°C to 23°C and a relative humidity of 50±5%. Irradiated pelleted food and autoclaved deionized water are provided ad libitum. Animals are identified by the use of individually numbered ear tags. N87 tumor fragments (appr 2 mm3) are implanted s.c. in the right flank of the animal. Before BT474 inoculation, 1.7 mg/90 day release 17β-estradiol pellets are placed s.c. BIIB021 is administered to animals bearing N87 stomach carcinoma tumors at doses of 31, 62.5, and 125 mg/kg, once daily, from Monday to Friday, for 5 weeks. Tumor dimensions are measured using calipers and tumor volumes are calculated using the equation for an ellipsoid sphere (l×w2)/2=mm3, where l and w refer to the larger and smaller dimensions collected at each measurement, respectively. Tumor volumes are measured and animals are weighed and monitored for toxicity at least twice weekly. P values are calculated using the two-tailed Student's t test to assess the difference in tumor volumes between control and treated groups. P<0.05 is considered significant. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 588.5±60.0 °C at 760 mmHg |
| Melting Point | 192-193℃ |
| Molecular Formula | C14H15ClN6O |
| Molecular Weight | 318.762 |
| Flash Point | 309.7±32.9 °C |
| Exact Mass | 318.099579 |
| PSA | 91.74000 |
| LogP | 1.66 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.711 |
| Storage condition | -20℃ |
| Hazard Codes | Xi |
|---|---|
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |