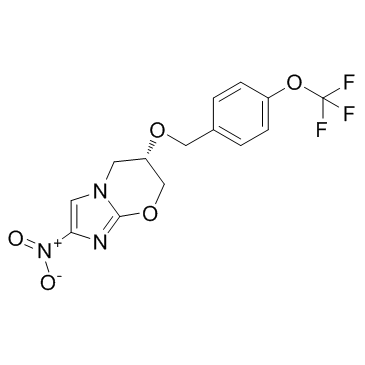
187235-37-6
| Name | (6S)-2-nitro-6-[[4-(trifluoromethoxy)phenyl]methoxy]-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine |
|---|---|
| Synonyms |
PA-824
5H-Imidazo[2,1-b][1,3]oxazine, 6,7-dihydro-2-nitro-6-[[4-(trifluoromethoxy)phenyl]methoxy]-, (6S)- (6S)-2-Nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine PA824 UNII-2XOI31YC4N (S)-6-(4-(trifluoromethoxy)benzyloxy)-2-nitro-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine 5H-Imidazo(2,1-b)(1,3)oxazine, 6,7-dihydro-2-nitro-6-((4-(trifluoromethoxy)phenyl)methoxy)-, (6S)- pretomanid |
| Description | Pretomanid (PA-824) is a small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis; the MIC values of PA-824 against a panel of MTB pan-sensitive and rifampin mono-resistant clinical isolates ranged from 0.015 to 0.25 ug/ml.IC50 value: 0.015 to 0.25 ug/ml (MICs) [1] |
|---|---|
| Related Catalog | |
| Target |
Tuberculosis. |
| In Vitro | Pretomanid (PA-824) exhibited a sub-micromolar minimal inhibitory concentration (MIC) against MTB, Although Pretomanid (PA-824) was not the most potent NAP against cultured MTB clinical isolates, it was the most active in infected mice when orally administered at 25 mg/kg. This indicated that Pretomanid (PA-824) might possess more desirable pharmacokinetic properties than the other more potent NAP compounds that we tested. Further studies in mice at 25, 50 and 100 mg kg-1 Pretomanid (PA-824) daily for 10 days resulted in reductions of mycobacterial burden in both spleen and lung tissues that were comparable to that of INH at 25 mg kg -1 [1]. Pretomanid (PA-824) showed significant activity at 2, 10, and 50 microg/ml, similar to that of metronidazole, in a dose-dependent manner. Pretomanid (PA-824) at 100 mg/kg in cyclodextrin/lecithin was as active as moxifloxacin at 100 mg/kg and isoniazid at 25 mg/kg and was slightly more active than rifampin at 20 mg/kg. Long-term treatment with Pretomanid (PA-824) at 100 mg/kg in cyclodextrin/lecithin reduced the bacterial load below 500 CFU in the lungs and spleen [2]. Pretomanid (PA-824) has no effect on the viability of M. leprae in all three models, consistent with the lack of the nitroimidazo-oxazine-specific nitroreductase, encoded by Rv3547 in the M. leprae genome, which is essential for activation of this molecule [3]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 462.3±55.0 °C at 760 mmHg |
| Melting Point | 150 °C |
| Molecular Formula | C14H12F3N3O5 |
| Molecular Weight | 359.257 |
| Flash Point | 233.4±31.5 °C |
| Exact Mass | 359.072906 |
| PSA | 91.33000 |
| LogP | 2.70 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.589 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P280-P304 + P340 + P312-P305 + P351 + P338-P337 + P313 |
| Hazard Codes | Xi |
| RIDADR | NONH for all modes of transport |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |