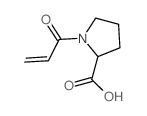
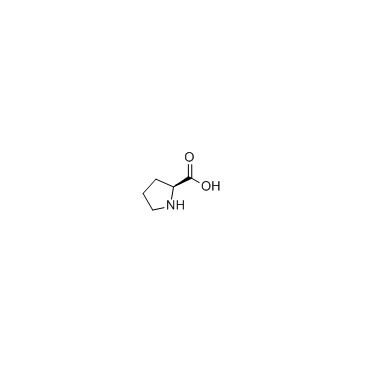
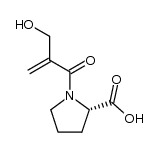
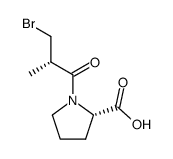
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UY0550000
-
CHEMICAL NAME :
-
1-Pyrrolidinecarboxylic acid, 1-(D-3-mercapto-2-methyl-1-propionyl)-, L-(S,S)-
-
CAS REGISTRY NUMBER :
-
62571-86-2
-
LAST UPDATED :
-
199609
-
DATA ITEMS CITED :
-
33
-
MOLECULAR FORMULA :
-
C9-H15-N-O3-S
-
MOLECULAR WEIGHT :
-
217.31
-
WISWESSER LINE NOTATION :
-
T5NTJ AVY1&1SH BVQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
10 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Vascular - BP lowering not characterized in autonomic section
-
REFERENCE :
-
AEMED3 Annals of Emergency Medicine. (American College of Emergency Physicians, 1125 Executive Circle, Irving, TX 75038) Volume(issue)/page/year: 20,1125,1991
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
16 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
JTCTDW Journal of Toxicology, Clinical Toxicology. (Marcel Dekker, 270 Madison Ave., New York, NY 10016) V.19- 1982- Volume(issue)/page/year: 28,379,1990
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
4 mg/kg/8D-I
-
TOXIC EFFECTS :
-
Behavioral - toxic psychosis
-
REFERENCE :
-
AJPSAO American Journal of Psychiatry. (American Psychiatric Assoc., Circulation Dept., 1400 K St., NW, Washington, DC 20005) V.78- 1921- Volume(issue)/page/year: 142,270,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
239 mg/kg/6W-I
-
TOXIC EFFECTS :
-
Sense Organs and Special Senses (Taste) - change in function Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Blood - normocytic anemia
-
REFERENCE :
-
AJMEAZ American Journal of Medicine. (Technical Pub., 875 Third Ave., New York, NY 10022) V.1- 1946- Volume(issue)/page/year: 71,493,1981
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2500 ug/kg/3D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - urine volume decreased Kidney, Ureter, Bladder - other changes
-
REFERENCE :
-
PGMJAO Postgraduate Medical Journal. (Blackwell Scientific Pub. Ltd., POB 88, Oxford, UK) V.1- 1925- Volume(issue)/page/year: 60,561,1984
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2679 ug/kg/5D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis)
-
REFERENCE :
-
IJMDAI Israel Journal of Medical Sciences. (POB 1435, Jerusalem 91013, Israel) V.1- 1965- Volume(issue)/page/year: 21,892,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
7143 ug/kg/2D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 294,91,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
12500 ug/kg/25D-I
-
TOXIC EFFECTS :
-
Blood - other hemolysis with or without anemia
-
REFERENCE :
-
CMAJAX Canadian Medical Association Journal. (Canadian Medical Assoc., POB 8650, Ottawa, ON K1G 0G8, Canada) V.1- 1911- Volume(issue)/page/year: 129,525,1983
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
14 mg/kg/2W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in tubules (including acute renal failure, acute tubular necrosis) Biochemical - Metabolism (Intermediary) - other
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 104,126,1986
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
1500 ug/kg/7W
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Vascular - BP lowering not characterized in autonomic section Lungs, Thorax, or Respiration - dyspnea
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 94,58,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - woman
-
DOSE/DURATION :
-
10 mg/kg/10D-I
-
TOXIC EFFECTS :
-
Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 294,91,1987
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
87 mg/kg/18W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes primarily in glomeruli Kidney, Ureter, Bladder - proteinuria Kidney, Ureter, Bladder - hematuria
-
REFERENCE :
-
AIMEAS Annals of Internal Medicine. (American College of Physicians, 4200 Pine St., Philadelphia, PA 19104) V.1- 1927- Volume(issue)/page/year: 112,550,1990
-
TYPE OF TEST :
-
LDLo - Lowest published lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Human - man
-
DOSE/DURATION :
-
2680 ug/kg/5D-I
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Nutritional and Gross Metabolic - changes in potassium
-
REFERENCE :
-
SMJOAV Southern Medical Journal. (Southern Medical Assoc., POB 2446, Birmingham, AL 35205) V.1- 1908- Volume(issue)/page/year: 86,1269,1993
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4245 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 14,297,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
>600 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,2439,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
554 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 14,297,1983
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2500 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
PCJOAU Pharmaceutical Chemistry Journal (English Translation). Translation of KHFZAN. (Plenum Pub. Corp., 233 Spring St., New York, NY 10013) No.1- 1967- Volume(issue)/page/year: 22,212,1988
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
>2400 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. (Yakugyo Jihosha, Inaoka Bldg., 2-36 Jinbo-cho, Kanda, Chiyoda-ku, Tokyo 101, Japan) V.1- 1959- Volume(issue)/page/year: 24,2439,1982
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
663 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
IYKEDH Iyakuhin Kenkyu. Study of Medical Supplies. (Nippon Koteisho Kyokai, 12-15, 2-chome, Shibuya, Shibuya-ku, Tokyo 150, Japan) V.1- 1970- Volume(issue)/page/year: 14,297,1983
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
>600 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
44XQA7 "Captopril and Hypertension, Collection of Papers presented at a Symposium, Princeton, N.J., 1979?," Case, David B., et al. eds., New York, Plenum Publishing, 1980 Volume(issue)/page/year: -,137,1980
-
TYPE OF TEST :
-
LD - Lethal dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
>1500 mg/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - nausea or vomiting
-
REFERENCE :
-
44XQA7 "Captopril and Hypertension, Collection of Papers presented at a Symposium, Princeton, N.J., 1979?," Case, David B., et al. eds., New York, Plenum Publishing, 1980 Volume(issue)/page/year: -,137,1980 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
45 gm/kg/13W-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - changes in bladder weight Blood - pigmented or nucleated red blood cells Blood - changes in erythrocyte (RBC) count
-
REFERENCE :
-
ARZNAD Arzneimittel-Forschung. Drug Research. (Editio Cantor Verlag, Postfach 1255, W-7960 Aulendorf, Fed. Rep. Ger.) V.1- 1951- Volume(issue)/page/year: 45,15,1995
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3 gm/kg/30D-I
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Liver - changes in liver weight Blood - changes in other cell count (unspecified)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6(Suppl 2),189,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
36500 mg/kg/1Y-C
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Blood - changes in serum composition (e.g. TP, bilirubin, cholesterol) Blood - changes in erythrocyte (RBC) count
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6(Suppl 2),215,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
9100 mg/kg/91D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes Skin and Appendages - dermatitis, other (after systemic exposure)
-
REFERENCE :
-
JTSCDR Journal of Toxicological Sciences. (Japanese Soc. of Toxicological Sciences, 4th Floor, Gakkai Center Bldg., 4-16, Yayoi 2-chome, Bunkyo-ku, Tokyo 113, Japan) V.1- 1976- Volume(issue)/page/year: 6(Suppl 2),247,1981
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
6300 mg/kg/28D-I
-
TOXIC EFFECTS :
-
Blood - other changes Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - other Enzymes
-
REFERENCE :
-
44XQA7 "Captopril and Hypertension, Collection of Papers presented at a Symposium, Princeton, N.J., 1979?," Case, David B., et al. eds., New York, Plenum Publishing, 1980 Volume(issue)/page/year: -,137,1980 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
228 mg/kg
-
SEX/DURATION :
-
male 43 week(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - breast development
-
REFERENCE :
-
BMJOAE British Medical Journal. (British Medical Assoc., BMA House, Tavistock Sq., London WC1H 9JR, UK) V.1- 1857- Volume(issue)/page/year: 296,1262,1988
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
48 gm/kg
-
SEX/DURATION :
-
male 30 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
REFERENCE :
-
YACHDS Yakuri to Chiryo. Pharmacology and Therapeutics. (Raifu Saiensu Shuppan K.K., 2-5-13, Yaesu, Chuo-ku, Tokyo 104, Japan) V.1- 1972- Volume(issue)/page/year: 13,7041,1985
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
RCOCB8 Research Communications in Chemical Pathology and Pharmacology. (PJD Pub. Ltd., P.O. Box 966, Westbury, NY 11590) V.1- 1970- Volume(issue)/page/year: 73,221,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
100 mg/kg
-
SEX/DURATION :
-
female 6-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
RCOCB8 Research Communications in Chemical Pathology and Pharmacology. (PJD Pub. Ltd., P.O. Box 966, Westbury, NY 11590) V.1- 1970- Volume(issue)/page/year: 73,221,1991
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
40 mg/kg
-
SEX/DURATION :
-
female 15-30 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
PSEBAA Proceedings of the Society for Experimental Biology and Medicine. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1903/04- Volume(issue)/page/year: 170,378,1982
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
8250 ug/kg
-
SEX/DURATION :
-
female 24-28 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 1,1256,1980 *** NIOSH STANDARDS DEVELOPMENT AND SURVEILLANCE DATA *** NIOSH OCCUPATIONAL EXPOSURE SURVEY DATA : NOES - National Occupational Exposure Survey (1983) NOES Hazard Code - X4863 No. of Facilities: 27 (estimated) No. of Industries: 1 No. of Occupations: 1 No. of Employees: 642 (estimated) No. of Female Employees: 346 (estimated)
|



![(2S)-1-[(2R)-3-hydroxy-2-methylpropanoyl]pyrrolidine-2-carboxylic acid structure](https://image.chemsrc.com/caspic/057/613256-52-3.png)