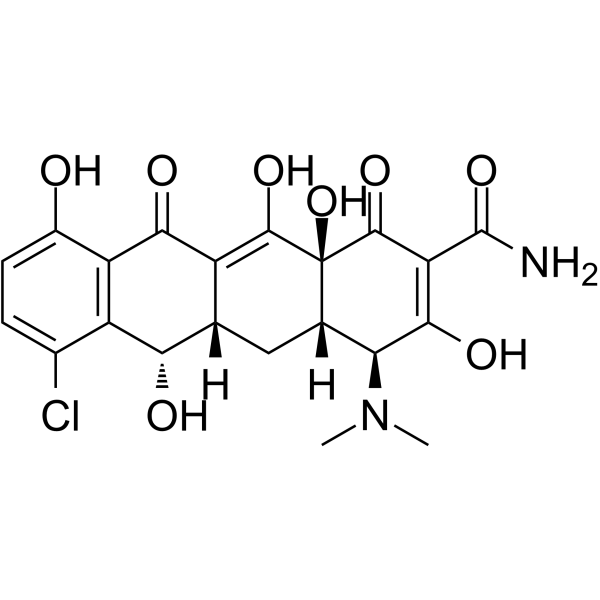
127-33-3
| Name | demeclocycline |
|---|---|
| Synonyms |
Ledermycin hydrochloride
7-chloro-6-demethyltetracycline 6-demethyl-7-chlorotetracycline (2E,4S,4aS,5aS,6S,12aS)-2-[Amino(hydroxy)methylene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5a,6,12a-tetrahydrotetracene-1,3,12(2H,4H,5H)-trione Elkamicina DMCTC (4aS)-7-Chlor-4c-dimethylamino-3,6t,10,12,12a-pentahydroxy-1,11-dioxo-(4ar,5ac,12ac)-1,4,4a,5,5a,6,11,12a-octahydro-naphthacen-2-carbonsaeure-amid Declomycin EINECS 204-834-8 Demeclocyclinum 7-chloro-4-dimethylamino-3,6,10,12,12a-pentahydroxy-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydro-naphthacene-2-carboxylic acid amide (2E,4S,4aS,5aS,6S,12aS)-2-[Amino(hydroxy)methylene]-7-chloro-4-(dimethylamino)-6,10,11,12a-tetrahydroxy-4a,5a,6,12a-tetrahydro-1,3,12(2H,4H,5H)-tetracenetrione 1,3,12(2H,4H,5H)-Naphthacenetrione, 2-(aminohydroxymethylene)-7-chloro-4-(dimethylamino)-4a,5a,6,12a-tetrahydro-6,10,11,12a-tetrahydroxy-, (2E,4S,4aS,5aS,6S,12aS)- Demethylchlortetracycline(DMCT) Demeclociclina Ledermycin domeclocycline (4S,4aS,5aS,6S,12aR)-7-chloro-4-(dimethylamino)-1,6,10,11,12a-pentahydroxy-3,12-dioxo-4a,5,5a,6-tetrahydro-4H-tetracene-2-carboxamide |
| Description | Demeclocycline is an orally active tetracycline antibiotic. Demeclocycline impairs protein synthesis by binding to the 30S ribosomal subunit to inhibit binding of aminoacyl tRNA. Demeclocycline shows anti-bacterial activitise to a wide variety of bacterial infections[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Demeclocycline (0-100 μM; 24 h) treatment reduces AQP2 abundance in mpkCCD cells[3]. Demeclocycline (10 μM; 24 h) treatment promotes the activity of monocytes and macrophages[4]. Demeclocycline (1-10 μM; 72 h) treatment directly affects the growth of brain tumorinitiating cells[4]. Western Blot Analysis[3] Cell Line: MpkCCD cells Concentration: 0-100 μM Incubation Time: 24 hours Result: Decreased AQP2 abundance in mpkCCD cells, with significant effects at 50 μM. Cell Viability Assay[4] Cell Line: mouse bone marrow derived macrophages and monocytes Concentration: 10 μM Incubation Time: 24 hours Result: Enhanced TNF-α production and modulated monocyte functions. Cell Viability Assay[4] Cell Line: brain tumorinitiating cells Concentration: 1, 5, and 10 μM Incubation Time: 72 hours Result: Inhibited cells growth in two ways: using monocytes as an intermediary, and directly by affecting the proliferation and sphere-forming capacity of brain tumorinitiating cells. |
| In Vivo | Demeclocycline (Intraperitoneal injection; 40 mg/kg; once daily; 48 h) treatment results in a significant reduction of hyponatremia and a significant correction of the hypoosmolality, and is not nephrotoxic[3]. Animal Model: Male Wistar rats induced with hyponatremia[3] Dosage: 40 mg/kg Administration: Intraperitoneal injection; 40 mg/kg; once daily; 48 hours Result: Increased urine volume, decreased urine osmolality, and caused a significantly increased fractional excretion of water. Animal Model: Male Wistar rats induced with hyponatremia[3] Dosage: 40 mg/kg Administration: Intraperitoneal injection; 40 mg/kg; once daily; 48 hours Result: Indicated the effect in the renal inner medulla for AQP2 and AC5/6 specifically, and not secondary toxicity effect. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 684.5±55.0 °C at 760 mmHg |
| Molecular Formula | C21H21ClN2O8 |
| Molecular Weight | 464.853 |
| Flash Point | 367.8±31.5 °C |
| Exact Mass | 464.098633 |
| PSA | 181.62000 |
| LogP | 0.57 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.761 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Precursor 0 | |
|---|---|
| DownStream 1 | |