20231-01-0
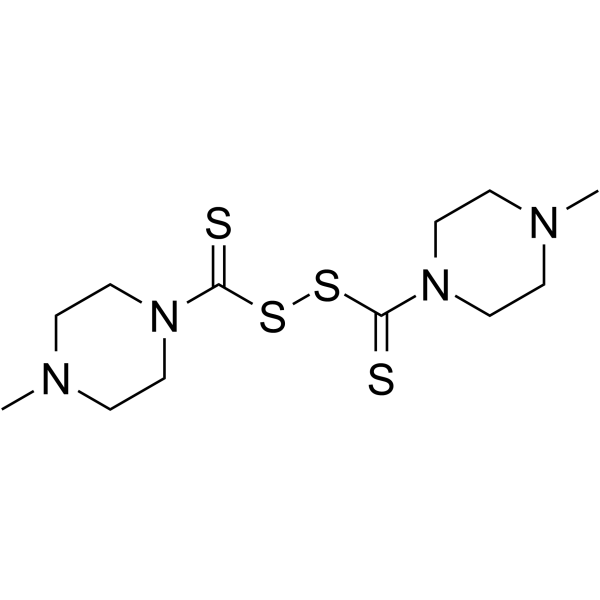
| Name | (4-methylpiperazine-1-carbothioyl)sulfanyl 4-methylpiperazine-1-carbodithioate |
|---|---|
| Synonyms |
Ewp 815
Piperazine,1,1'-(dithiodicarbonothioyl)bis(4-methyl bis((4-methylpiperazino)thiocarbamoyl)disulfide MFCD01729803 Bis((4-methyl-1-piperazinyl)thiocarbonyl)disulfide Bis-<(4-methyl-1-piperazinyl)-thiocarbonyl>disulfid DISULFIDE,BIS((4-METHYL-1-PIPERAZINYL)THIOCARBONYL) 4-Methylpiperazino-thiuramdisulfide bis-(4-methyl-piperazine-1-carbothioyl)-disulfane 4,4'-dimethyl-1,1'-(2,3-dithia-butanedithioyl)-bis-piperazine di-N-methylpiperazinethiuramdisulfide |
| Description | EWP 815 is a disulfiram analogue, is a potent inhibitor of Ins(1,4)P2 phosphatase and Ins(1,4,5)P3 5-phosphatase. EWP 815 also inhibits enzyme dopamine β-hydroxylase activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Ins(1,4)P2 phosphatase; Ins(1,4,5)P3 5-phosphatase[1]; dopamine β-hydroxylase[2] |
| In Vitro | EWP 815 inhibits thyrotropin-releasing hormone (TRH; 0.03 mM)-stimulated inositol phospholipid breakdown without affecting basal breakdown rates in prelabelled GH3 cells with IC50s of 83 mM (soluble enzymes) and 71 mM (particulate enzymes), respectively[1]. EWP 815 (30 μM; 1 h) exerts higher inhibition on Ins(1,4)P2 phosphatase rather than Ins(1,4,5)P3 5-phosphatase with the mean [3H]Ins(1,4)P2/mean [3H]InsP recovery ratio of 2.6[1]. EWP 815 (6, 8 μM; 10 min) inhibits dephosphorylation of [3H]Ins(1,4,5)P3 5-phosphatase in particulate and soluble fractions with IC50s of 6 μM and 8 μM, respectively[1]. EWP 815 (3-300 μM; 30 min) suppresses thyrotropin-releasing hormone (TRH)-stimulated (100 nM) inositol phospholipid production by 30% (100 μM) and 1.8% (300 μM), respectively, without affecting Ins(1,4,5)P3 binding[1]. |
| In Vivo | EWP 815 (50 mg/kg; i.p.; 30 min before killed) inhibits the enzyme dopamine β-hydroxylase activity in mice[2]. Animal Model: Female mice (20 g)[2] Dosage: 50 mg/kg Administration: Intraperitoneal injection; 30 min before killed Result: Intraperitoneal injection; 30 min before killedInhibited β-hydroxylase of 3H-α-methyldopamine. Reduced the amount of 3H-α-Me-NA by 12%. |
| References |
| Density | 1.339g/cm3 |
|---|---|
| Boiling Point | 450.5ºC at 760 mmHg |
| Melting Point | 142ºC |
| Molecular Formula | C12H22N4S4 |
| Molecular Weight | 350.59000 |
| Flash Point | 226.3ºC |
| Exact Mass | 350.07300 |
| PSA | 127.74000 |
| LogP | 1.18400 |
| Vapour Pressure | 2.62E-08mmHg at 25°C |
| Index of Refraction | 1.673 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S24/25 |
|---|---|
| HS Code | 2933599090 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |