3105-97-3
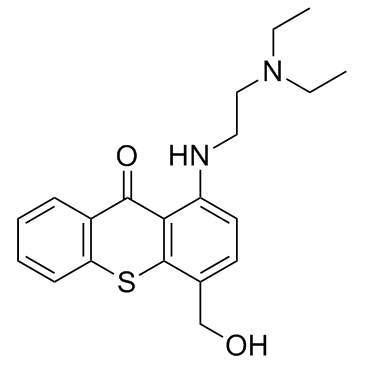
| Name | hycanthone |
|---|---|
| Synonyms |
lucanthonemetabolite
1-(2-diethylaminoethylamino)-4-methylol-thioxanthen-9-one 1-[(2-[DIETHYLAMINO]ETHYL)AMINO]-4-[HYDROXYMETHYL]-9H-THIOXANTHEN-9-ONE ETRENOL 1-((2-(diethylamino)ethyl)amino)-4-(hydroxymethyl)thioxanthen-9-one hycanthon Win 249-33 Etrenol(mesylate) 1-((2-(diethylamino)ethyl)amino)-4-(hydroxymethyl)-9h-thioxanthen-9-on HYCANTHONE |
| Description | Hycanthone is an effective antischistosomai drug. |
|---|---|
| Related Catalog | |
| Target |
Parasite[1] |
| In Vitro | Hycanthone at 20 mg/mL or more is progressively more detrimental to cell viability. Results reveal that increased concentrations of Hycanthone, ranging from 0.1 to 10 μg/mL, progressively reduces viral interferon yields as much as 73% compare to that of controls[2]. |
| In Vivo | Results show that the incorporation of tritiated thymidine into TCA-precipitable material of adult sensitive worms undergo a progressive decrease after treatment with Hycanthone. Immature worms are totally unaffected by Hycanthone at all times tested. Male worms treated with Hycanthone show signs of a possible partial recovery from the initial low levels of incorporation. The incorporation of tritiated leucine by drug-sensitive worms treated with Hycanthone is inhibited by 40 to 50% in the first four days after treatment. Results show that, 7 days after Hycanthone treatment, both ribosomal RNA species are reduced by at least 80% with respect to untreated worms, with some indication of a possible accumulation of heavier precursor molecules[1]. |
| Cell Assay | Appropriate quantities of Hycanthone (1 to 100 μg) in 10 mL maintenance medium are added to plastic flasks (75 cm2) containing approximately 3×107 LLC-MK2 cells in monolayer, which are then incubated at 35°C for 24 h. Maintenance medium is decanted, and 2 mL influenza virus is added onto cell monolayers and incubated at 35°C for 2 h. The multiplicity of infection is approximately 1.0. Inoculum is removed and 10 mL maintenance medium is added to each flask, which is then incubated at 35°C for 24 h. Supernatant fluid is decanted, centrifuged at 100,000 g for 1 h, dialyzed against HCI-KCI buffer (pH 2.0) at 4°C for 24 h, and then dialyzed against two changes of phosphate-buffered saline (pH 7.1) at 4°C for 24 h. Fluids are passed through filters to obtain sterile preparations. Samples are stored at -80°C until assayed for interferon activity[2]. |
| Animal Admin | Female outbred Swiss albino mice used as definitive hosts weigh 18 to 20 g at the time of infection. Hycanthone is administered at 0.01 mL/g body weight intramuscularly by splitting the dose into the two hind legs, so that each mouse receives 80 mg/kg body weight of the free base. Treatments are usually performed during the 8th week after infection[1]. |
| References |
| Density | 1.25g/cm3 |
|---|---|
| Boiling Point | 570.5ºC at 760mmHg |
| Melting Point | approx 143ºC |
| Molecular Formula | C20H24N2O2S |
| Molecular Weight | 356.48200 |
| Flash Point | 298.9ºC |
| Exact Mass | 356.15600 |
| PSA | 80.81000 |
| LogP | 3.73370 |
| Index of Refraction | 1.658 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | T: Toxic; |
|---|---|
| Risk Phrases | 45-46-20/21/22 |
| HS Code | 2932999099 |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![9H-Thioxanthen-9-one, 4-[(acetyloxy)methyl]-1-[[2- (diethylamino)ethyl]amino]- structure](https://image.chemsrc.com/caspic/087/3612-72-4.png)
![[1-(2-diethylaminoethylamino)-9-oxo-thioxanthen-4-yl]methyl N-phenylcarbamate structure](https://image.chemsrc.com/caspic/011/3612-74-6.png)