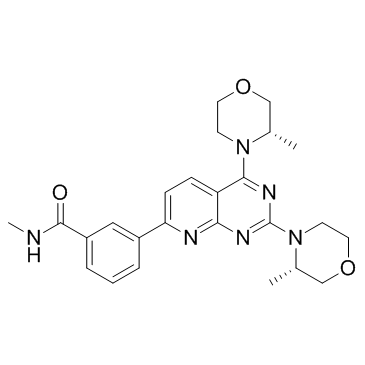
1009298-59-2
| Name | 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide |
|---|---|
| Synonyms |
vistusertib
AZD2014 |
| Description | Vistusertib (AZD2014) is an ATP competitive mTOR inhibitor with an IC50 of 2.81 nM. AZD2014 inhibits both mTORC1 and mTORC2 complexes. |
|---|---|
| Related Catalog | |
| Target |
mTOR:2.81 nM (IC50) mTORC1 mTORC2 PI3Kα:3.766 μM (IC50) Autophagy |
| In Vitro | The inhibitory effects of Vistusertib (AZD2014) are measured against isolated recombinant mTOR enzyme (IC50 of 2.81 nM) as well as in cellular assays measuring both mTORC1 and mTORC2 activities. In MDAMB468 cells, Vistusertib (AZD2014) decreases the phosphorylation of the mTORC1 substrate ribosomal protein S6 (Ser235/236) with a mean IC50 value of 210 nM and the mTORC2 substrate AKT (Ser473) with a mean IC50 value of 78 nM[1]. |
| In Vivo | Vistusertib (AZD2014) induces dose-dependent tumor growth inhibition in several xenograft and primary explant models. The antitumor activity of Vistusertib (AZD2014) is associated with modulation of both mTORC1 and mTORC2 substrates, consistent with its mechanism of action. The pharmacokinetics of Vistusertib (AZD2014) in mice is tested upon administration of doses between 7.5 and 15 mg/kg. A dose-dependent increase in Cmax and AUC is observed following single dose and repeat dosing of AZD2014: Cmax range from 1 to 16 μM and AUC range from 220 to 5,042 μM·h across this dose range. The pharmacodynamic effect of Vistusertib (AZD2014) against an mTORC1 biomarker (phosphorylation of S6) and an mTORC2 biomarker (phosphorylation of AKT) is assessed in SCID mice bearing MCF7 xenografts following administration of 3.75, 7.5, and 15 mg/kg AZD2014. There is a good relationship between the drug plasma concentrations and biomarker levels (estimated p-AKT IC50 of 0.119 μM total, 53% SE, and estimated p-S6 IC50 0.392 μM, 28.8% SE)[1]. |
| Animal Admin | Mice[1] MCF7 experiments: 5×106 MCF7 cells are injected s.c. in a volume of 0.1 mL in male SCID mice and are randomized into control and treatment groups when tumor size reach 0.2 cm3. Vistusertib (AZD2014) is dissolved in captisol, and diluted to a final captisol concentration of 30% (w/v). Vistusertib (AZD2014) is administered by oral gavage (0.1 mL/10 g body weight). The control group receive vehicle only. Tumor volumes (measured by calliper), animal body weight and condition are recorded twice weekly for the duration of the study. The tumor volume is calculated (taking length to be the longest diameter across and width to be the corresponding perpendicular diameter) using the formula: (length×width)×√(length×width)×(π/6). |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Molecular Formula | C25H30N6O3 |
| Molecular Weight | 462.544 |
| Exact Mass | 462.237946 |
| PSA | 96.20000 |
| LogP | 0.28 |
| Index of Refraction | 1.607 |
| Storage condition | 2~8°C |
| Hazard Codes | Xi |
|---|---|
| HS Code | 29339900 |