124020-27-5
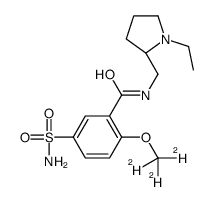
| Name | N-[[(2S)-1-ethylpyrrolidin-2-yl]methyl]-5-sulfamoyl-2-(trideuteriomethoxy)benzamide |
|---|---|
| Synonyms |
S-Sulpiride-d3
Levopraid-d3 Levosulpiride-d3 L-Sulpiride-d3 Sulpid-d3 Levopride-d3 Levobren-d3 (-)-N-[(1-Ethyl-2-pyrrolidinyl)methyl]-5-sulfamoyl-o-anisamide-d3 (-)-Sulpiride-d3 |
| Description | Levosulpiride-d3 (RV-12309-d3) is the deuterium labeled Levosulpiride. Levosulpiride (RV-12309) is the (S)-enantiomer of sulpiride, which is a D2 receptor a antagonist, an atypical antipsychotic drug of the benzamide class[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C15H20D3N3O4S |
|---|---|
| Molecular Weight | 344.44400 |
| Exact Mass | 344.16000 |
| PSA | 113.60000 |
| LogP | 2.85050 |