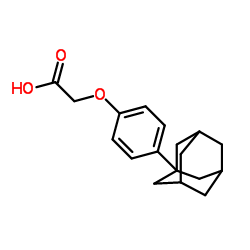
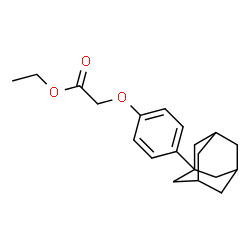
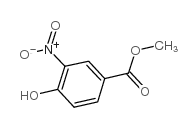
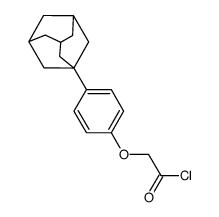
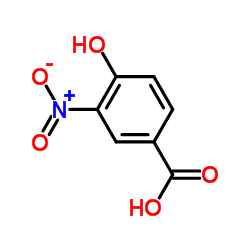
934593-90-5
| Name | methyl 3-[[2-[4-(2-adamantyl)phenoxy]acetyl]amino]-4-hydroxybenzoate |
|---|---|
| Synonyms |
Hypoxia Inducible Factor-1alpha Inhibitor
methyl 4-hydroxy-3-({[4-(tricyclo[3.3.1.1]dec-1-yl)phenoxy]acetyl}amino)benzoate Benzoic acid, 4-hydroxy-3-[[2-(4-tricyclo[3.3.1.1]dec-1-ylphenoxy)acetyl]amino]-, methyl ester Methyl 3-[({4-[(3s,5s,7s)-adamantan-1-yl]phenoxy}acetyl)amino]-4-hydroxybenzoate Methyl 3-({[4-(adamantan-1-yl)phenoxy]acetyl}amino)-4-hydroxybenzoate CAY10585 LW6 |
| Description | LW6 is a novel HIF-1 inhibitor with an IC50 of 4.4 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 4.4 μM (HIF-1)[1] |
| In Vitro | LW6 decreases HIF-1α protein expression without affecting HIF-1β expression. LW6 affects the stability of the HIF-1α protein. LW6 promotes the degradation of wild type HIF-1α, but not of a DM-HIF-1α with modifications of P402A and P564A, at hydroxylation sites in the oxygen-dependent degradation domain. LW6 induces the expression of von Hippel-Lindau (VHL), which interacts with prolyl-hydroxylated HIF-1α for proteasomal degradation. In the presence of LW6, knockdown of VHL does not abolish HIF-1α protein accumulation, indicating that LW6 degraded HIF-1α via regulation of VHL expression[2]. In MDCKII-BCRP cells overexpressing BCRP, LW6 enhances significantly the cellular accumulation of mitoxantrone, a BCRP substrate. LW6 also down-regulates BCRP expression at concentrations of 0.1-10 µM[3]. LW6 inhibits the expression of HIF 1α induced by hypoxia in A549 cells at 20 mM, independently of the von Hippel Lindau protein. LW6 induces hypoxia selective apoptosis together with a reduction in the mitochondrial membrane potential[4]. |
| In Vivo | In mice carrying xenografts of human colon cancer HCT116 cells, LW6 demonstrates strong anti-tumor efficacy in vivo and causes a decrease in HIF-1α expression in frozen-tissue immunohistochemical staining[2]. |
| Cell Assay | Inhibition of HIF-1a is assayed by a reporter assay using dualluciferase reporter assay system. HCT116 cells in 75-90% confluence are transiently co-transfected with pGL3-HRE-luciferase plasmid containing six copies of HREs from human VEGF genes and pRLSV40 encoding firefly renilla luciferase and incubated for 24 h. Cells are treated with LW6 or 17-AAG for 16 h before report assay. Luciferase activity is integrated over a 10 second period and measured using a luminometer[2]. |
| Animal Admin | Mice: The mice receive the following treatments using a dosing vehicle solution, containing 10% dimethylacetamide, 10% Cremophor EL and 80% of sodium carbonate buffer (pH 10), by intraperitoneal (i.p.) injection: group1(control group; six mice), vehicle solution; group2 (six mice), LW6 at a dose of 10 and 20mg/kg (QD); and group 3 (six mice), topotecan at a dose of 2mg/kg, (Q2D), which is the dose and dosing schedule that showed more than 60% inhibition of growth of HCT116 tumors. The treatments are continued for 13 days[2]. |
| References |
[3]. Song JG, et al. Discovery of LW6 as a new potent inhibitor of breast cancer resistance protein. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 647.4±55.0 °C at 760 mmHg |
| Molecular Formula | C26H29NO5 |
| Molecular Weight | 435.512 |
| Flash Point | 345.3±31.5 °C |
| Exact Mass | 435.204559 |
| PSA | 88.35000 |
| LogP | 6.49 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.637 |
| HS Code | 2924299090 |
|---|
|
~84% 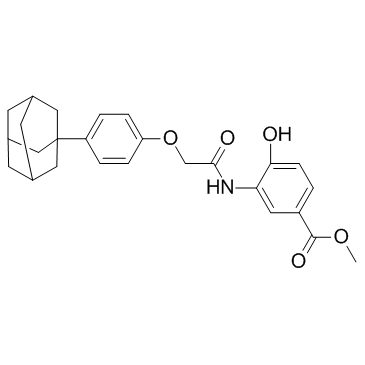
934593-90-5 |
| Literature: DONGGUK UNIVERSITY INDUSTRY-ACADEMIC COOPERATION FOUNDATION; KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY; Lee, Kyeong; Won, Mi Sun; Kim, Hwan Mook; Park, Song Kyu; Lee, Kiho; Lee, Ki Hoon; Lee, Chang Woo; Lee, Jung Joon; Chung, Kyung Sook; Kim, Bo Kyung; Jin, Yinglan; Lee, Seung-hee Patent: US2013/237542 A1, 2013 ; Location in patent: Paragraph 0216; 0217; 0218 ; |
|
~86% 
934593-90-5 |
| Literature: KOREA RESEARCH INSTITUTE OF BIOSCIENCE AND BIOTECHNOLOGY Patent: WO2008/4798 A1, 2008 ; Location in patent: Page/Page column 30 ; |
|
~% 
934593-90-5 |
| Literature: Lee, Kyeong; Lee, Jeong Hyung; Boovanahalli, Shanthaveerappa K.; Jin, Yinglan; Lee, Mijeoung; Jin, Xuejun; Kim, Jin Hwan; Hong, Young-Soo; Lee, Jung Joon Journal of Medicinal Chemistry, 2007 , vol. 50, # 7 p. 1675 - 1684 |
|
~% 
934593-90-5 |
| Literature: Lee, Kyeong; Lee, Jeong Hyung; Boovanahalli, Shanthaveerappa K.; Jin, Yinglan; Lee, Mijeoung; Jin, Xuejun; Kim, Jin Hwan; Hong, Young-Soo; Lee, Jung Joon Journal of Medicinal Chemistry, 2007 , vol. 50, # 7 p. 1675 - 1684 |
|
~% 
934593-90-5 |
| Literature: Nakamura, Hiroyuki; Yasui, Yuka; Ban, Hyun Seung Journal of Organometallic Chemistry, 2013 , vol. 747, p. 189 - 194 |
|
~% 
934593-90-5 |
| Literature: Korea Research Institute of Bioscience and Biotechnology; Dongguk University Industry-Academic Cooperation Foundation; WON, Mi-Sun; KIM, Hwan-Mook; LEE, Kyeong; PARK, Song-Kyu; LEE, Ki-Ho; LEE, Chang-Woo; LEE, Jung-Joon; CHUNG, Kyung-Sook; KIM, Bo-Kyung; JIN, Ying-Lan Patent: WO2012/53768 A2, 2012 ; Location in patent: Page/Page column 14-15 ; |
|
~% 
934593-90-5 |
| Literature: Nakamura, Hiroyuki; Yasui, Yuka; Ban, Hyun Seung Journal of Organometallic Chemistry, 2013 , vol. 747, p. 189 - 194 |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |