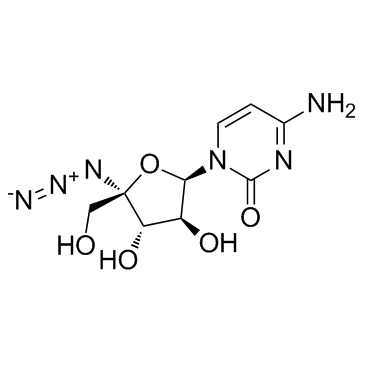
876708-03-1
| Name | 2(1H)-Pyrimidinone, 4-amino-1-(4-C-azido-β-D-arabinofuranosyl) |
|---|---|
| Synonyms |
RO 9187
4-amino-1-[(2R,3S,4S,5R)-5-azido-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]pyrimidin-2-one RO-9187 |
| Description | RO-9187 is a potent inhibitor of HCV virus replication with an IC50 of 171 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 171 nM (HCV)[1] |
| In Vitro | RO-9187 is excellent substrates for deoxycytidine kinase and is phosphorylated with efficiencies up to 3-fold higher than deoxycytidine. RO-9187 inhibits RNA synthesis by HCV polymerases from either HCV genotypes 1a and 1b or containing S96T or S282T point mutations with similar potencies, suggesting no cross-resistance with either R1479 (4′-azidocytidine) or 2′-C-methyl nucleosides. The formation of RO-9187-TP increased in a time- and dose-dependent manner. The maximal triphosphate concentration at 24 h is 87 pmol/106 cells with half-maximal triphosphate formation achieved at 12 μM RO-9187[1]. |
| In Vivo | Plasma exposures of RO-9187 in rats increase in a dose-dependent manner between 10 and 2000 mg/kg after oral dosing. Plasma concentrations of 1.4 and 26 μM (390 and 7454 ng/mL) are achieved in rats and dogs at the 10 mg/kg dose level, respectively. Plasma concentrations up to 57 μM are achieved in rats dosed with 2000 mg/kg/day[1]. |
| Animal Admin | Rats: A 2-week oral range finding toxicity study is performed with RO-9187 and ribavirin in Hanover-Wistar rats. Five male and five female rats in each of five treatment groups are administered once daily doses of vehicle, 200, 600, or 2000 mg/kg RO-9187 or 200 mg/kg ribavirin by oral gavage for 14 days[1]. |
| References |
| Molecular Formula | C9H12N6O5 |
|---|---|
| Molecular Weight | 284.22900 |
| Exact Mass | 284.08700 |
| PSA | 180.58000 |
| Storage condition | 2-8℃ |