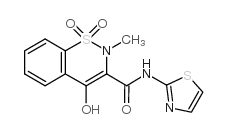
34042-85-8
| Name | 4-hydroxy-2-methyl-1,1-dioxo-N-(1,3-thiazol-2-yl)-1λ6,2-benzothiazine-3-carboxamide |
|---|---|
| Synonyms |
Sudoxicamum
4-hydroxy-2-methyl-N-(2-thiazole)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide N-(2-thiazolyl)-4-hydroxy-2-methyl-2H,1,2-benzothiazine-3-carboxamide 1,1-dioxide Sudoxicamum [INN-Latin] 2H-1,2-Benzothiazine-3-carboxamide,4-hydroxy-2-methyl-N-2-thiazolyl-,1,1-dioxide 4-hydroxy-2-methyl-N-(2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide EINECS 251-808-7 SUDOXICAM |
| Description | Sudoxicam is a reversible and orally active COX antagonist and a non-steroidal anti-inflammatory drug (NSAID) from the enol-carboxamide class. Sudoxicam has potent anti-inflammatory, anti-edema and antipyretic activity[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
COX[3] |
| In Vitro | Sudoxicam demonstrates NADPH-dependent covalent binding to human liver microsomes. With addition of glutathione (GSH) in microsomal incubations, about half of the covalent incorporation of Sudoxicam is blocked by addition of GSH[1]. Metabolite identification studies on [14C]-Sudoxicam in NADPH-supplemented human liver microsomes indicated that the primary route of metabolism involved a P450-mediated thiazole ring scission to the corresponding acylthiourea metabolite (S3), a well-established pro-toxin[1]. In vitro, Sudoxicam underwent the oxidative thiazole-open biotransformation, resulting in the formation of an acylthiourea and the subsequent hydroxylated metabolite[3]. |
| In Vivo | Sudoxicam (1-10 mg/kg; oral administration; daily; for 7 days; rats) treatment effective reduces plasma inflammation units, reduces the swelling of inflamed hind-paws and restores toward normal the daily gain in body weight[2]. In the intact rat, Sudoxicam significantly inhibited edema formation at doses as low as 0.1 mg/kg, p.o[2]. Sudoxicam inhibits the erythema caused by ultraviolet irradiation in the guinea pig. Sudoxicam (3.3 mg/kg, i.p.) is capable of counteracting the pyrexia induced by the intraperitoneal injection of typhoid/paratyphoid vaccine in rats, maintaining body temperature about that of uninjected control rats[2]. The plasma half-life of Sudoxicam ranged between 8 hours (monkey), 13 hours (rat), and 60 hours (dog)[2]. Animal Model: Rats injected with ,i>M. butyrieum[2] Dosage: 1 mg/kg, 3.3 mg/kg, 10 mg/kg Administration: Oral administration; daily; for 7 days Result: Were both effective in reducing plasma inflammation units, in reducing the swelling of inflamed hind-paws. |
| References |
[3]. Zhi-Yi Zhang. Sudoxicam. Handbook of Metabolic Pathways of Xenobiotics. September 2014. |
| Density | 1.675g/cm3 |
|---|---|
| Melting Point | 240-243 °C (dec.) |
| Molecular Formula | C13H11N3O4S2 |
| Molecular Weight | 337.37400 |
| Exact Mass | 337.01900 |
| PSA | 136.22000 |
| LogP | 2.73420 |
| Index of Refraction | 1.741 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 5 | |
|---|---|
| DownStream 0 | |