43229-80-7
| Name | Formoterol fumarate |
|---|---|
| Synonyms |
Formoterol fumarate
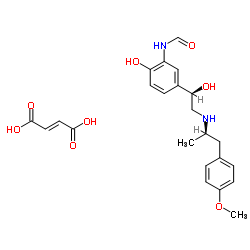
N-{2-Hydroxy-5-[(1R)-1-hydroxy-2-{[(2R)-1-(4-methoxyphenyl)propan-2-yl]amino}ethyl]phenyl}formamide (2E)-but-2-enedioate (1:1) N-{2-Hydroxy-5-[(1R)-1-hydroxy-2-{[(2R)-1-(4-methoxyphenyl)-2-propanyl]amino}ethyl]phenyl}formamide (2E)-2-butenedioate (1:1) (2E)-But-2-endisäure--N-{2-hydroxy-5-[(1R)-1-hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino}ethyl]phenyl}formamid(1:1) (R*,R*)-(±)-N-[2-Hydroxy-5-[1-hydroxy-2-[[2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]formamide (E)-2-Butenedioate (2:1) (Salt) CGP 25827A eformoterol Atock Formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2E)-2-butenedioate (1:1) (salt) [(2S)-2-(3-formamido-4-hydroxyphenyl)-2-hydroxyethyl]-[(2S)-1-(4-methoxyphenyl)propan-2-yl]azanium Oxeze Formoterol Hemifumarate BD 40 A N-{2-hydroxy-5-[(1R)-1-hydroxy-2-{[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino}ethyl]phenyl}formamide (2E)-but-2-enedioate (salt) (±)-2'-Hydroxy-5'-[(RS)-1-hydroxy-2-[[(RS)-p-methoxy-a-methylphenethyl]amino]ethyl]formanilide (E)-2-Butenedioate (2:1) (Salt) acide (2E)-but-2-ènedioïque - N-{2-hydroxy-5-[(1R)-1-hydroxy-2-{[(1R)-2-(4-méthoxyphényl)-1-méthyléthyl]amino}éthyl]phényl}formamide (1:1) Aformoterol Formoterol (Fumarate) |
| Description | Formoterol fumarate(Foradil) is a potent, selective and long-acting β2-adrenoceptor agonist.IC50 Value: 2.1 nM in pregnant C3H/HeN strain mice[5]Target: β2 receptorBudesonide/formoterol in a single inhaler for both maintenance and reliever therapy is now an established therapeutic option for management of inadequately controlled asthma[4].in vitro:. The long-acting β(2)-agonist formoterol and the glucocorticoid dexamethasone significantly reduced HRV-induced ERK phosphorylation, Fra-1, and MMP-9 expression in BEAS-2B cells[3].in vivo: compared the bronchodilatory effects of inhaled budesonide/formoterol (dose: 200 μg and 12 μg respectively) combination with budesonide (200 μg)/salbutamol (200 μg) administered by metered dose inhaler and spacer in children of 5-15 years with mild acute exacerbation of asthma [Modified Pulmonary Index Score (MPIS) between 6-8] in this double-blind, randomized controlled trial. The primary outcome was FEV1 (% predicted) in the two groups at 1, 5, 15, 30, 60 min after administration of the study drug[1]. Fifteen randomized, placebo-controlled clinical trials including COPD patients were evaluated: indacaterol 75 μg once daily (n = 2 studies), indacaterol 150 μg once daily (n = 5), indacaterol 300 μg once daily (n = 4), FOR/BUD 9/160 μg twice daily (n = 2), FOR/BUD 9/320 μg twice daily (n = 2), SAL/FP 50/500 μg twice daily (n = 4), and SAL/FP 50/250 μg twice daily (n = 1)[2].Clinical trial: Effects of Mometasone Furoate/Formoterol Combination Versus Mometasone Furoate Alone in Persistent Asthmatics (Study P04073) . Phage3 |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 603.2ºC at 760mmHg |
|---|---|
| Melting Point | 138-140ºC |
| Molecular Formula | C19H24N2O4.1/2C4H4O4 |
| Molecular Weight | 460.477 |
| Flash Point | 318.6ºC |
| Exact Mass | 460.184570 |
| PSA | 165.42000 |
| LogP | 3.03490 |
| Storage condition | 2-8°C |
| Water Solubility | Slightly soluble in water, soluble in methanol, slightly soluble in 2-propanol, practically insoluble in acetonitrile. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S36/37 |
|---|---|
| RIDADR | UN 3249 |
| RTECS | LQ2988000 |
| Packaging Group | II |
| Hazard Class | 6.1(b) |
| HS Code | 2924299090 |
|
~% 
43229-80-7 |
| Literature: Hett, Robert; Fang, Qun K.; Gao, Yun; Wald, Stephen A.; Senanayake, Chris H. Organic Process Research and Development, 1998 , vol. 2, # 2 p. 96 - 99 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2924299090 |
|---|---|
| Summary | 2924299090. other cyclic amides (including cyclic carbamates) and their derivatives; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
