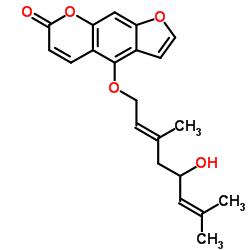
88206-46-6
| Name | (E)-4-((5-Hydroxy-3,7-dimethylocta-2,6-dien-1-yl)oxy)-7H-furo[3,2-g]chromen-7-one |
|---|---|
| Synonyms |
4-[(2E)-5-hydroxy-3,7-dimethylocta-2,6-dienoxy]furo[3,2-g]chromen-7-one
4-{[(2E)-5-hydroxy-3,7-dimethylocta-2,6-dien-1-yl]oxy}-7H-furo[3,2-g]chromen-7-one 4-{[(2E)-5-Hydroxy-3,7-dimethyl-2,6-octadien-1-yl]oxy}-7H-furo[3,2-g]chromen-7-one 7H-Furo[3,2-g][1]benzopyran-7-one, 4-[[(2E)-5-hydroxy-3,7-dimethyl-2,6-octadien-1-yl]oxy]- 7H-Furo(3,2-g)(1)benzopyran-7-one, 4-((5-hydroxy-3,7-dimethyl-2,6-octadienyl)oxy)-, (E)- Notopterol |
| Description | Notopterol is a coumarin extracted from N. incisum. Notopterol induces apoptosis and has antipyretic, analgesic and anti-inflammatory effects. Notopterol is used for acute myeloid leukemia (AML)[1]. |
|---|---|
| Related Catalog | |
| In Vitro | notopterol (5, 10, 20, 40, 60 μM; for 48 hours) promotes cell apoptosis in a dose-dependent manner[1]. notopterol (10, 20, 40, 60 and 80 μM; 24-120 hours) inhibits the proliferation of HL-60 cells in a concentration-dependent manner[1]. Notopterol (5, 10, 20 and 40 μM; for 48 hours) induces G0/G1 phase arrest associated with cell-cycle proteins in HL-60 cells[1]. Notopterol (5, 10, 20 and 40 μM; for 48 hours) increases the expression of Bax and decreased the expression of Bcl-2 and Mcl-1 in HL-60 cells after 48-hr treatment. Moreover, Notopterol also promotes the cleavage of caspase 9, caspase 3 and PARP[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 546.7±50.0 °C at 760 mmHg |
| Molecular Formula | C21H22O5 |
| Molecular Weight | 354.396 |
| Flash Point | 284.4±30.1 °C |
| Exact Mass | 354.146729 |
| PSA | 72.81000 |
| LogP | 4.25 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.599 |
| Safety Phrases | 24/25 |
|---|---|
| HS Code | 2932999099 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2932999099 |
|---|---|
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |