81226-60-0
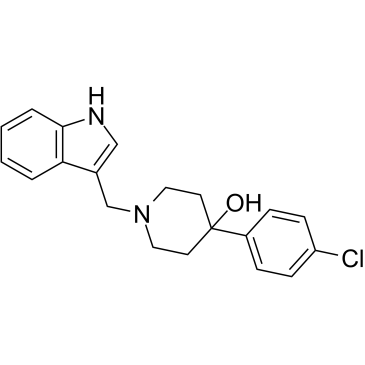
| Name | 4-(4-chlorophenyl)-1-(1H-indol-3-ylmethyl)piperidin-4-ol |
|---|---|
| Synonyms |
4-(4-Chlorophenyl)-1-(1H-indol-3-ylmethyl)-4-piperidinol
L 741,626 1-(indol-3-ylmethyl)-4-hydroxy-4-(p-chlorophenyl)piperidine 4-Piperidinol,4-(4-chlorophenyl)-1-(1H-indol-3-ylmethyl) 3-(4-(4-Chlorophenyl-4-hydroxypiperidino)methyl)indole |
| Description | L-741626 is a selective D2 dopamine receptor antagonist, with the Ki values of 2.4, 100 and 220 nM for human D2, D3 and D4 receptors respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.4 nM (human D2 receptor), 100 nM (human D3 receptor), 220 nM (human D4 receptor)[1] |
| In Vitro | Intrinsic activities in a functional assay using inhibition of quinpirole stimulation of mitogenesis in human dopamine D2 or D3 receptors transfected into Chinese hamster ovary (CHO) cells, L-741626 is prepared by literature methods (Ki (D2)=11.2 nM) and displays a D3/D2 and D4/D2 selectivity ratio of 15-fold and 136-fold, respectively. In the functional assay L-741626 is a potent antagonist (EC50 (D2)=4.46 nM) with some D2 selectivity (EC50 (D3)=90.4 nM)[2]. |
| In Vivo | L-741626 (1.0 mg/kg; i.h.) is effective at shifting to the right the pramipexole dose-response curve in pramipexole-trained male Sprague Dawley rats[3]. Coadministrating Cocaine with the D2 antagonist L-741626 (3 mg/kg; i.p.; 15 min before Cocaine) for 5 days reduces the Cocaine-induced increase in microglial TNF-α production in adult mice[4]. |
| References |
| Density | 1.311g/cm3 |
|---|---|
| Boiling Point | 548.8ºC at 760 mmHg |
| Molecular Formula | C20H21ClN2O |
| Molecular Weight | 340.84700 |
| Flash Point | 285.7ºC |
| Exact Mass | 340.13400 |
| PSA | 39.26000 |
| LogP | 4.24280 |
| Index of Refraction | 1.686 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 
81226-60-0 |
| Literature: John Wyeth and Brother Limited Patent: US4358456 A1, 1982 ; |
|
~95% 
81226-60-0 |
| Literature: Vangveravong, Suwanna; McElveen, Elizabeth; Taylor, Michelle; Xu, Jinbin; Tu, Zhude; Luedtke, Robert R.; Mach, Robert H. Bioorganic and Medicinal Chemistry, 2006 , vol. 14, # 3 p. 815 - 825 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |