317318-84-6
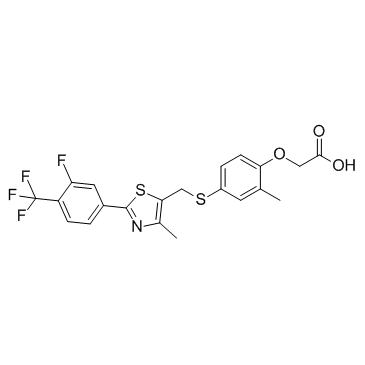
| Name | 2-[4-[[2-[3-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methylsulfanyl]-2-methylphenoxy]acetic acid |
|---|---|
| Synonyms |
Acetic acid, 2-[4-[[[2-[3-fluoro-4-(trifluoromethyl)phenyl]-4-methyl-5-thiazolyl]methyl]thio]-2-methylphenoxy]-
gw 0742 {4-[({2-[3-Fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl}methyl)sulfanyl]-2-methylphenoxy}acetic acid GW0742 |
| Description | GW0742 is a high affinity PPAR β/δ agonist with an IC50 of 1 nM for human PPARδ, and EC50s of 1 nM, 1.1 μM and 2 μM for human PPARδ, PPARα, and PPARγ, respectively. |
|---|---|
| Related Catalog | |
| Target |
PPARδ:1 nM (EC50) PPARα:1.1 μM (EC50) PPARγ:2 μM (EC50) |
| In Vitro | GW0742 is a potent PPARβ and PPARδ agonist, with an IC50 of 1 nM for human PPARδ, and EC50s of 1 nM, 1.1 μM and 2 μM for human PPARδ, PPARα, and PPARγ respectively[1]. GW0742 (100 μM) activates human PPARα and mouse PPARβ in MCF-7 cells. GW0742 (100 μM) significantly reduces low-KCl-induced apoptosis of cerebellar granule neurons. GW0742 shows no obvious inherent toxicity on cerebellar granule neuronal cells after treatment of 3-100 μM for 24 h, but induces increased cell death at 100 μM after 48 hr of treatment. Moreover, GW0742 (100 μM) increases c-Jun expression in cerebellar granule neuron cultures observed at 6 hr[2]. GW0742 (1 μM) induces PPARδ protein in neonatal rat cardiomyocytes. GW0742 also raises mRNA levels of long-chain acyl-CoA dehydrogenase (LCAD), very long-chain acyl-CoA dehydrogenase (VLCAD), acyl-CoA oxidase 1 (ACOX1), uncoupling protein 3 (UCP3), malonyl-CoA decarboxylase (MCD), and pyruvate dehydrogenase kinase 4 (PDK4) in neonatal rat cardiomyocytes[4]. |
| In Vivo | GW0742 (0.3 mg/kg, i.p.) reduces intensity masson-trichrome staining, and attenuates the histological signs in bleomycin instillatio (BLEO)-induced lung injury of mice. GW0742 (0.3 mg/kg, i.p.) also causes a reduction of the BLEO-induced loss body weight, and a decrease of myeloperoxidase (MPO) activity. GW0742 shows significant inhibition of TNF-a and IL-1β in instilled-mice. GW0742 prevents bleomycin-induced IkB-a degradation, reduces the levels of NF-kB p65 in the lung, and decreases iNOS and p-ERK expression in BLEO-induced mice[3]. GW0742 (5 mg/kg/day, i.v.) increases PPARδ protein level in the heart of rats. GW0742 also induces the increase in LCAD, VLCAD, and ACOX1 in the heart of rats[4]. |
| Cell Assay | The PPARβ activator GW0742 and the RXR activator 9-cis-retinoic acid are dissolved in DMSO. The final DMSO concentration des not exceed 0.5% v/v, and this concentration is used in control wells. For each culture plate, one row of wells is treated with 500 μM glutamate. These wells serve as a positive control and for normalisation of data. Cell death (toxicity) is assessed by using an assay designed to measure lactate dehydrogenase (LDH) release[2]. |
| Animal Admin | Male CD mice (25-35 g) are housed in a controlled environment and provided with standard rodent chow and water. Mice are randomized into four experimental groups: bleomycin-treated group: mice are subjected to lung injury induced by intratracheal instillation of bleomycin and treated daily via intraperitoneal injection with vehicle of GW0742 (10% dimethylsulfoxide (OMSO, 1 mL/kg), 1 h after BLEO instillation (n = 15). GW0742 group: identical to bleomycin-treated group but mice are treated daily with GW0742 (0.3 mg/kg, 1h after BLEO instillation) via intraperitoneal injection (n = 15). Sham-operated mice + vehicle group: animals are subjected to the identical surgical procedure but receive intratracheal instillation of saline (0.9%) instead of BLEO and are treated daily with the vehicle of GW0742 (10% dimethylsulfoxide (DMSO), 1 mL/kg, i.p.), 1 h after saline instillation (n = 15). Sham-operated mice + GW0742 group: identical to sham + vehicle group but mice are treated daily with GW0742 (0.3 mg/kg, 1 h after saline instillation) via intraperitoneal injection (n = 15)[3]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 591.5±60.0 °C at 760 mmHg |
| Melting Point | 134.5-135.5 °C |
| Molecular Formula | C21H17F4NO3S2 |
| Molecular Weight | 471.488 |
| Flash Point | 311.5±32.9 °C |
| Exact Mass | 471.058594 |
| PSA | 112.96000 |
| LogP | 6.57 |
| Appearance | solid | white |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.609 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: >5 mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |