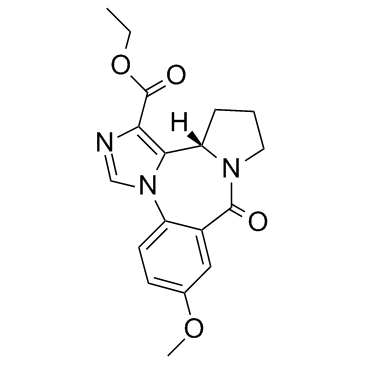
130477-52-0
| Name | L-655,708,11,12,13,13a-Tetrahydro-7-methoxy-9-oxo-9H-imidazo[1,5-a]pyrrolo[2,1-c][1,4]benzodiazepine-1-carboxylicacid,ethylester |
|---|---|
| Synonyms |
l-655,708
L-655708 |
| Description | L-655708 is a potent α5 subunit-selective GABAA receptor inverse agonist (Ki = 0.45 nM).IC50: 0.45 nM (Ki)Target: GABAin vitro: L-655708 is a potent, selective inverse agonist for the benzodiazepine site of GABAA receptors containing the α5 subunit (Ki = 0.45 nM). Displays 50-100-fold selectivity over GABAA receptors containing α1, α2, α3 orα6 subunits in combination with β3 and γ2. Enhances LTP in a mouse hippocampal slice model and increases spatial learning, without displaying proconvulsant activity.in vivo: L-655708 at 0.7 mg/kg, administered intraperitoneally, would result in 60-70% occupancy of α5 GABAA receptors with limited binding to α1, α2, and α3 subunit-containing GABAA receptors and no significant off-target behavioral effects, such as sedation and motor impairment.[1] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.42g/cm3 |
|---|---|
| Boiling Point | 584.4ºC at 760mmHg |
| Molecular Formula | C18H19N3O4 |
| Molecular Weight | 341.36100 |
| Flash Point | 307.2ºC |
| Exact Mass | 341.13800 |
| PSA | 73.66000 |
| LogP | 2.28620 |
| Vapour Pressure | 1.21E-13mmHg at 25°C |
| Index of Refraction | 1.675 |
| Storage condition | 2-8℃ |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |