383432-38-0
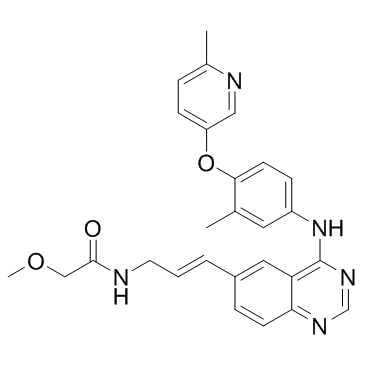
| Name | E-2-Methoxy-N-(3-{4-[3-methyl-4-(6-methyl-pyridin-3-yloxy)-phenylamino]-quinazolin-6-yl}-allyl)-acetamide |
|---|---|
| Synonyms |
Kumarin
2-methoxy-N-(3-(4-((3-methyl-4-((6-methyl-3-pyridinyl)oxy)phenyl)amino)-6-quinazolinyl)-2-propenyl)acetamide Rattex CUMARIN COUMARINE chromen-2-one 2-Methoxy-N-[(2E)-3-[4-[[3-methyl-4-[(6-methyl-3-pyridinyl)oxy]phenyl]amino]-6-quinazolinyl]-2-propen-1-yl]acetamide 2-Methoxy-N-{(2E)-3-[4-({3-methyl-4-[(6-methyl-3-pyridinyl)oxy]phenyl}amino)-6-quinazolinyl]-2-propen-1-yl}acetamide Acetamide, 2-methoxy-N-[(2E)-3-[4-[[3-methyl-4-[(6-methyl-3-pyridinyl)oxy]phenyl]amino]-6-quinazolinyl]-2-propen-1-yl]- CP-724714 |
| Description | CP-724,714 is a potent, selective inhibitor of HER2/ErbB2 with IC50 of 10 nM, >640-fold selectivity against EGFR, InsR, IRG-1R, PDGFR, VEGFR2, Abl, Src, c-Met etc. Phase 2.IC50 value: 10 nM [1]Target: HER2/ErbB2in vitro: CP-724,714 is marked selectively against EGFR with IC50 of 6.4 μM. CP-724,714 is >1,000-fold less potent for IR, IGF-1R, PDGFRβ, VGFR2, abl. Src, c-Met c-jun NH2-terminal kinase (JNK)-2, JNK-3, ZAP-70, cyclin-dependent kinase (CDK)-2, and CDK-5. CP-724,714 potently reduces the EGF-induced autophosphorylation of the chimera containing the erbB2 kinase domain with IC50 of 32 nM, but is markedly less potent against EGFR in transfected NIH3T3 cells. CP-724,714 sensitively inhibits the proliferation of erbB2-amplified cells including BT-474 and SKBR3, with IC50 of 0.25 and 0.95 μM. CP-724,714 induces the accumulation of cells in G1 phase and a marked reduction in S-phase in BT-474 cells at 1 μM [1]. CP-724,714 likely exerts its hepatotoxicity via both hepatocellular injury and hepatobiliary cholestatic mechanisms. CP-724,714 displays inhibition of cholyl-lysyl fluorescein and taurocholate (TC) efflux into canaliculi in cryopreserved and fresh cultured human hepatocytes, respectively. CP-724,714 inhibits TC transport in membrane vesicles expressing human bile salt export pump with IC50 of 16 μM and inhibits the major efflux transporter in bile canaliculi, MDR1, with IC50 of ~28 μM [2]. in vivo: CP-724,714 (25 mg/kg) is rapidly absorbed after p.o. administration and causes reduction of tumor erbB2 receptor phosphorylation after dosing in FRE-erbB2 or BT-474 xenografts. CP-724,714 induces apoptosis in FRE-erbB2 xenograft–bearing (s.c.) mice and shows 50% tumor growth inhibition at 50 mg/kg, without weight loss or mortality. CP-724,714 also has great antitumor activity in MDA-MB-453, MDA-MB-231, LoVo (colon), and Colo-205 (colon) xenografts. Furthermore, CP-724,714 (30 or 100 mg/kg) reduces the extracellular signal–regulated kinase and Akt phosphorylation in BT-474 xenografts [1]. |
|---|---|
| Related Catalog | |
| Target |
ErbB2:10 nM (IC50) |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 687.3±55.0 °C at 760 mmHg |
| Molecular Formula | C27H27N5O3 |
| Molecular Weight | 469.535 |
| Flash Point | 369.5±31.5 °C |
| Exact Mass | 469.211395 |
| PSA | 98.26000 |
| LogP | 4.05 |
| Vapour Pressure | 0.0±2.1 mmHg at 25°C |
| Index of Refraction | 1.663 |
| Storage condition | 2-8℃ |
| Precursor 10 | |
|---|---|
| DownStream 0 | |


![(6-iodoquinazolin-4-yl)-[3-methyl-4-(6-methylpyridin-3-yloxy)phenyl]amine hydrochloride structure](https://image.chemsrc.com/caspic/089/537705-10-5.png)