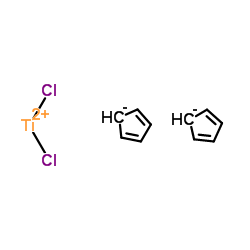
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
XR2050000
-
CHEMICAL NAME :
-
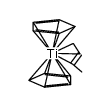
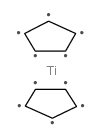
Titanium, dichloro-di-pi-cyclopentadienyl-
-
CAS REGISTRY NUMBER :
-
1271-19-8
-
LAST UPDATED :
-
199710
-
DATA ITEMS CITED :
-
24
-
MOLECULAR FORMULA :
-
C10-H10-Cl2-Ti
-
MOLECULAR WEIGHT :
-
249.00
-
WISWESSER LINE NOTATION :
-
L50J 0-TI-GG- 0L50J
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
25 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
60 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Behavioral - changes in motor activity (specific assay)
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
180 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
6 gm/kg/14D-I
-
TOXIC EFFECTS :
-
Kidney, Ureter, Bladder - other changes in urine composition Blood - pigmented or nucleated red blood cells Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
4030 mg/kg/13W-I
-
TOXIC EFFECTS :
-
Cardiac - changes in heart weight Endocrine - changes in thymus weight Nutritional and Gross Metabolic - weight loss or decreased weight gain
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
26 gm/kg/2Y-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Gastrointestinal - tumors
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
720 mg/kg/2Y-I
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Blood - lymphoma, including Hodgkin's disease Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
75 mg/kg
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Blood - lymphoma, including Hodgkin's disease Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
900 mg/kg/2Y-I
-
TOXIC EFFECTS :
-
Tumorigenic - neoplastic by RTECS criteria Musculoskeletal - tumors Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TD - Toxic dose (other than lowest)
-
ROUTE OF EXPOSURE :
-
Intramuscular
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
430 mg/kg/81W-I
-
TOXIC EFFECTS :
-
Tumorigenic - equivocal tumorigenic agent by RTECS criteria Blood - lymphoma, including Hodgkin's disease Tumorigenic - tumors at site of application
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
MUTATION DATA
-
TYPE OF TEST :
-
Morphological transformation
-
TEST SYSTEM :
-
Rodent - hamster Embryo
-
DOSE/DURATION :
-
100 ug/L
-
REFERENCE :
-
JJIND8 JNCI, Journal of the National Cancer Institute. (Washington, DC) V.61-79, 1978-87. For publisher information, see JNCIEQ. Volume(issue)/page/year: 67,1303,1981
|




![[2-(N,N-dimethylaminomethyl)phenyl]di-n-butyltin(IV) chloride structure](https://image.chemsrc.com/caspic/268/215306-19-7.png)
![[2-(N,N-dimethylaminomethyl)phenyl]diphenyltin(IV) chloride structure](https://image.chemsrc.com/caspic/390/342371-85-1.png)