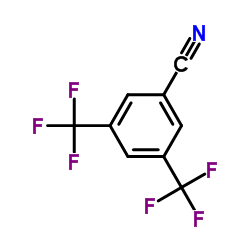
175205-09-1
| Name | 5-[3,5-Bis(trifluoromethyl)phenyl]tetrazole |
|---|---|
| Synonyms |
MFCD00052524
5-[3,5-bis(trifluoromethyl)phenyl]-2H-tetrazole |
| Description | KG-548 is an ARNT/TACC3 disruptor and a HIF-1α inhibitor. KG-548 directly interferes with ARNT/TACC3 complex formation by competing with TACC3 for binding to the ARNT PAS-B domain. ARNT is the aryl hydrocarbon receptor nuclear translocator, also known as HIF-β[1][2]. |
|---|---|
| Related Catalog | |
| Target |
HIF-β[1]; HIF-1α[2] |
| In Vitro | HIF is a heterodimer of two bHLH-PAS (basic Helix Loop Helix-Per-ARNT-Sim) subunits, including a HIF-α paralog (HIF-1α, -2α, -3α) and aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIF-β)[1]. KG-548 (0-250 μM; 16 h for over night) exhibits the great reduction of ARNT/CCC complex formation for both coactivators, and also disrupts ARNT2 PAS-B/TACC3 interactions[1]. KG-548 (0-2 mM; 16 h for over night) dose-dependently breaks up the (320 μM) ARNT PAS-B/TACC3 complex in vitro and in cell lysate with an IC50 value of 25 μM[1]. KG-548 significantly inhibits lactate production of glycolysis on in FaDu hypopharyngeal carcinoma cells[2]. Western Blot Analysis[1] Cell Line: HEK293T cell lysates Concentration: 0, 5, 50, 100, 250, 500 μM and 1 mM, 2 mM Incubation Time: 16 hours Result: Weakened the ARNT/TACC3 interaction from 5 μM to 500 μM dose-dependently, and decreased the protein intense of ARNT associated with immunoprecipitated TACC3 protein by 82% (500 μM), 59% (1 mM), 43% (2 mM), respectively. |
| Melting Point | 177-180°C |
|---|---|
| Molecular Formula | C9H4F6N4 |
| Molecular Weight | 282.14500 |
| Exact Mass | 282.03400 |
| PSA | 54.46000 |
| LogP | 2.90430 |
| Storage condition | 2-8°C |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302 + H332-H315-H319 |
| Precautionary Statements | P210-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi |
| Risk Phrases | 25-36/37/38 |
| Safety Phrases | 26-45 |
| RIDADR | UN 1648 3 / PGII |
| HS Code | 2933990090 |
| Flash Point(F) | 41 °F |
| Flash Point(C) | 5 °C |
|
~60% 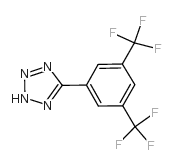
175205-09-1 |
| Literature: Sigma-Aldrich Co. Patent: US2006/247431 A1, 2006 ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |