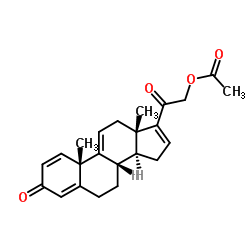
37413-91-5
| Name | [2-[(8S,10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,12,14,15-hexahydrocyclopenta[a]phenanthren-17-yl]-2-oxoethyl] acetate |
|---|---|
| Synonyms |
21-(Acetyloxy)pregna-1,4,9(11),16-tetraene-3,20-dione
EINECS 253-497-3 16-tetraene-3,20-dione 21-acetate 21-hydroxypregna-1,4,9(11) 21-acetoxy-pregna-1,4,9(11),16-tetraene-3,20-dione 3,20-Dioxopregna-1,4,9(11),16-tetraen-21-yl acetate 21-Acetoxy-pregna-1,4,9(11),16-tetraen-3,20-dion Pregna-1,4,9(11),16-tetraene-3,20-dione, 21-(acetyloxy)- 21-hydroxy-pregna-1,4,9(11),16-tetraene-3,20-dione 21-acetate 2-((8S,10S,13S,14S)-10,13-dimethyl-3-oxo-6,7,8,10,12,13,14,15-octahydro-3H-cyclopenta[a]phenanthren-17-yl)-2-oxoethyl acetate 21-Acetoxypregna-1,4,9(11),16-tetraene-3,20-dione |
| Description | 21-Acetoxypregna-1,4,9(11),16-tetraene-3,20-dione is an intermediate of delta 9,11 steroids synthesis, for example, Vamorolone (HY-109017). The delta 9,11 steroids are modifications of glucocorticoids and has anti-inflammatory properties. The delta 9,11 steroids are agents for protection against cell damage (lipid peroxidation) and inhibition of neovascularization[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Δ9,11 derivatives are designed and developed to stably incorporate into cell membranes and inhibit lipid peroxidation without glucocorticoid or mineralocorticoid activities, thus avoiding side effects associated with traditional corticosteroids[1]. VBP15 acts as a lead compound due to potent NF-κB inhibition and GR translocation similar to prednisone and dexamethasone, lack of transactivation properties, and good bioavailability[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 534.6±50.0 °C at 760 mmHg |
| Molecular Formula | C23H26O4 |
| Molecular Weight | 366.450 |
| Flash Point | 233.0±30.2 °C |
| Exact Mass | 366.183105 |
| PSA | 60.44000 |
| LogP | 3.48 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.585 |
|
~% 
37413-91-5 |
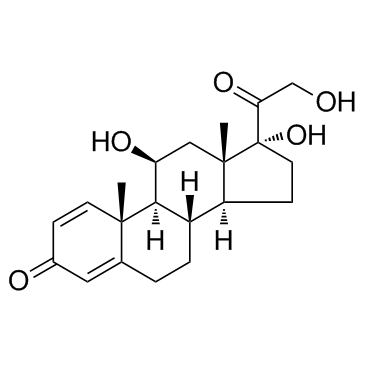
| Literature: Schaub et al. Journal of the American Chemical Society, 1959 , vol. 81, p. 4962,4966 |
|
~% 
37413-91-5 |
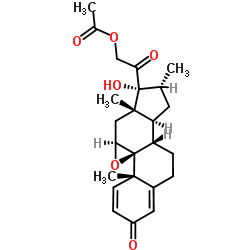
| Literature: Hulcoop, David G.; Shapland, Peter D.P. Steroids, 2013 , vol. 78, # 12-13 p. 1281 - 1287 |
|
~% 
37413-91-5 |
| Literature: Am.Cyanamid Co. Patent: US2864834 , 1957 ; |
| Precursor 3 | |
|---|---|
| DownStream 1 | |