517-44-2
| Name | sennidine b |
|---|---|
| Synonyms |
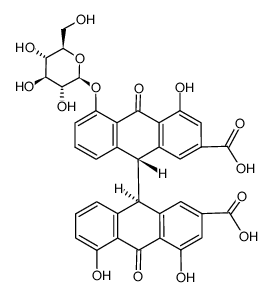
(9R,9'S)-4,4',5,5'-Tetrahydroxy-10,10'-dioxo-9,9',10,10'-tetrahydro-9,9'-bianthracene-2,2'-dicarboxylic acid
1,4-Dichlorobutane-2,3-acetonide meso-4,5-Bis-chlormethyl-2,2-dimethyl-dioxolan Sennidin B [9,9'-Bianthracene]-2,2'-dicarboxylic acid, 9,9',10,10'-tetrahydro-4,4',5,5'-tetrahydroxy-10,10'-dioxo-, (9R,9'S)- |
| Description | Sennidin B, a stereoisomer isolated from the leaves of Cassia angustifolia, has lower activity than Sennidin A. Sennidin A inhibits HCV NS3 helicase, with an IC50 of 0.8 μM. Sennidin A induces phosphorylation of Akt and glucose transporter 4 (GLUT4) translocation. Sennidin A stimulates the glucose incorporation [1][2]. |
|---|---|
| Related Catalog | |
| References |
[2]. Daigo Abe, et al. Sennidin stimulates glucose incorporation in rat adipocytes. Life Sciences. 2006. |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 801.8±65.0 °C at 760 mmHg |
| Molecular Formula | C30H18O10 |
| Molecular Weight | 538.458 |
| Flash Point | 452.6±30.8 °C |
| Exact Mass | 538.090027 |
| PSA | 189.66000 |
| LogP | 8.24 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.806 |
|
~% 
517-44-2 
517-44-2 |
| Literature: Hattori; Kim; Motoike; Kobashi; Namba Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 4 p. 1338 - 1346 |
|
~% 
517-44-2
Detail
|
| Literature: Hattori; Kim; Motoike; Kobashi; Namba Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 4 p. 1338 - 1346 |
|
~% 
517-44-2 
517-44-2 |
| Literature: Hattori; Kim; Motoike; Kobashi; Namba Chemical and Pharmaceutical Bulletin, 1982 , vol. 30, # 4 p. 1338 - 1346 |
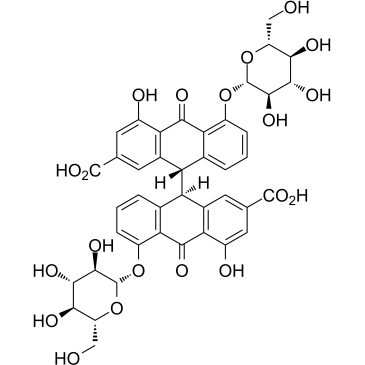
| Precursor 3 | |
|---|---|
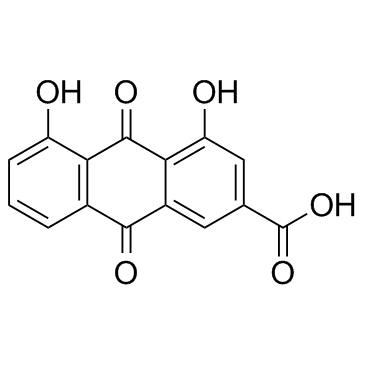
| DownStream 2 | |