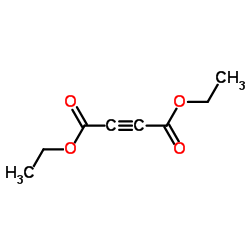
52089-62-0
| Name | diethyl 2,2,3,3-tetradeuteriobutanedioate |
|---|---|
| Synonyms |
Diethyl (H)butanedioate
Diethyl succinate-2,2,3,3-d4 Diethyl butanedioate-2,2,3,3-d4 Butanedioic-d acid, diethyl ester MFCD00075229 |
| Description | Diethyl succinate-d4 is the deuterium labeled Diethyl succinate[1]. Diethyl succinate (Diethyl Butanedioate) is used at physiological pH and crosses biological membranes, incorporates into cells in tissue culture and is metabolized by the TCA cycle. Diethyl succinate is known to be non-toxic and used in fragrances and flavoring[2]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 218.4±8.0 °C at 760 mmHg |
| Melting Point | -20ºC(lit.) |
| Molecular Formula | C8H10D4O4 |
| Molecular Weight | 178.219 |
| Flash Point | 90.6±0.0 °C |
| Exact Mass | 178.114319 |
| PSA | 52.60000 |
| LogP | 1.26 |
| Vapour Pressure | 0.1±0.4 mmHg at 25°C |
| Index of Refraction | 1.423 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
|
~92% 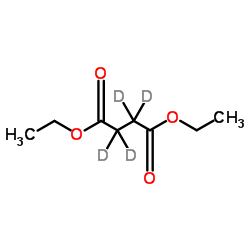
52089-62-0 |
| Literature: Sajiki, Hironao; Kurita, Takanori; Esaki, Hiroyoshi; Aoki, Fumiyo; Maegawa, Tomohiro; Hirota, Kosaku Organic Letters, 2004 , vol. 6, # 20 p. 3521 - 3523 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |