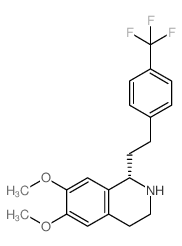
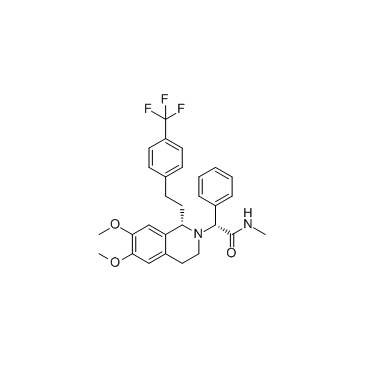
871224-64-5
| Name | (R)-2-((R)-6,7-dimethoxy-1-(4-(trifluoromethyl)phenethyl)-3,4-dihydroisoquinolin-2(1H)-yl)-N-methyl-2-phenylacetamide |
|---|---|
| Synonyms |
(2R)-2-[(1S)-6,7-Dimethoxy-1-{2-[4-(trifluoromethyl)phenyl]ethyl}-3,4-dihydro-2(1H)-isoquinolinyl]-N-methyl-2-phenylacetamide
(R)-2-{(S)-6,7-dimethoxy-1-[2-(4-trifluoromethylphenyl)ethyl]-3,4-dihydro-1H-isoquinolin-2-yl}-N-methyl-2-phenylacetamide Almorexant [inn] [3H]-Almorexant 2(1H)-Isoquinolineacetamide, 3,4-dihydro-6,7-dimethoxy-N-methyl-α-phenyl-1-[2-[4-(trifluoromethyl)phenyl]ethyl]-, (αR,1S)- AlMorexant HCl 2(1H)-Isoquinolineacetamide, 3,4-dihydro-6,7-dimethoxy-N-methyl-α-phenyl-1-[2-[4-(trifluoromethyl)phenyl]ethyl]-, (αR,1R)- (2R)-2-((1S)-6,7-Dimethoxy-1-{2-(4-(trifluoromethyl)phenyl)ethyl}-3,4-dihydroisoquinolin-2(1H)-yl)-N-methyl-2-phenylacetamide Almorexant (2R)-2-{(1S)-6,7-dimethoxy-1-[2-(4-trifluoromethyl-phenyl)-ethyl]-3,4-dihydro-1H-isoquinolin-2-yl}-N-methyl-2-phenyl-acetamide ((2R)-2-((1S)-6,7-dimethoxy-1-(2-(4-trifluoromethylphenyl)-ethyl)-3,4-dihydro-1H-isoquinolin-2-yl)-N-methyl-2-phenylacetamide) (2R)-2-[(1R)-6,7-Dimethoxy-1-{2-[4-(trifluoromethyl)phenyl]ethyl}-3,4-dihydro-2(1H)-isoquinolinyl]-N-methyl-2-phenylacetamide |
| Description | Almorexant(ACT078573) is a potent and competitive dual orexin 1 receptor (OX1)/orexin 2 receptor (OX2) antagonist with Ki values of 1.3 and 0.17 nM for OX1 and OX2, respectively.IC50 value: 1.3/0.7 nM(OX1/OX2 receptor) [1] [2]Target: Dual OX!/OX2 receptorin vitro: [(3)H]Almorexant bound to a single saturable site on hOX(1) and hOX(2) with high affinity (K(d) of 1.3 and 0.17 nM, respectively. In Schild analyses using the [(3)H]inositol phosphates assay, almorexant acted as a competitive antagonist at hOX(1) and as a noncompetitive-like antagonist at hOX(2). In binding kinetic analyses, [(3)H]almorexant had fast association and dissociation rates at hOX(1), whereas it had a fast association rate and a remarkably slow dissociation rate at hOX(2) [1]. in vivo: During the 12-h dark period after dosing, ALM(Almorexant) exacerbated cataplexy in TG mice and increased nonrapid eye movement sleep with heightened sleep/wake fragmentation in both genotypes. ALM showed greater hypnotic potency in WT mice than in TG mice. The 100 mg/kg dose conferred maximal promotion of cataplexy in TG mice and maximal promotion of REM sleep in WT mice. In TG mice, ALM (30 mg/ kg) paradoxically induced a transient increase in active wakefulness [3]. Almorexant 200 mg showed significantly less 'Drug Liking' than both zolpidem doses (p < 0.01), and almorexant 400 mg had smaller effects than zolpidem 20 mg (p < 0.05), while almorexant 1,000 mg was not different from either zolpidem dose [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 620.4±55.0 °C at 760 mmHg |
| Molecular Formula | C29H31F3N2O3 |
| Molecular Weight | 512.563 |
| Flash Point | 329.0±31.5 °C |
| Exact Mass | 512.228699 |
| PSA | 50.80000 |
| LogP | 5.89 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.554 |
|
~% 
871224-64-5 |
| Literature: WO2009/83899 A2, ; Page/Page column 28 ; WO2009/83903 A1, ; Page/Page column 28 ; |
|
~% 
871224-64-5 |
| Literature: WO2005/118548 A1, ; Page/Page column 28 ; WO 2005/118548 A1 |
|
~% 
871224-64-5 |
| Literature: WO2005/118548 A1, ; Page/Page column 29 ; WO 2005/118548 A1 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |