1018946-38-7
| Name | 2-(3,4-dimethoxyphenyl)-5-(3-methoxypropyl)-1-benzofuran |
|---|---|
| Synonyms |
DWK-1339
2-(3,4-dimethoxy-phenyl)-5-(3-methoxy-propyl)-benzofuran UNII-WN8TEM4Q1F Benzofuran,2-(3,4-dimethoxyphenyl)-5-(3-methoxypropyl) |
| Description | DWK-1339 is an orally active and blood-brain-barrier-permeable Aβ-aggregation inhibitor, used in the research of Alzheimer's disease. |
|---|---|
| Related Catalog | |
| Target |
Amyloid-β[1] |
| In Vitro | DWK-1339 (MDR-1339) is an Aβ-aggregation inhibitor, and shows no significant inhibition a panel of CYP isozymes, while it slightly inhibits CYP2C8 (IC50, 31.4 μM). DWK-1339 (3.1-50 μM) dose-dependently blocks the formation of Aβ aggregates, and disaggregates Aβ fibrils. DWK-1339 (1.5-10 μM) also protects cells from this Aβ-induced toxicity[1]. |
| In Vivo | DWK-1339 (0.1-10 mg/kg, p.o.) dose-dependently restores the passive avoidance responses in mice models of Alzheimer's disease (AD), with an ED50 of 0.19 mg/kg. DWK-1339 (30 and 100 mg/kg, p.o. daily for 8 weeks) significantly improves spontaneous alternation, and reduces the Aβ1-40 and Aβ1-42 levels in APP/PS1 mice[1]. |
| Cell Assay | HT22 cells, a murine cell line of hippocampal origin, are grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 5% penicillin/streptomycin. At the outset, 90% confluent cells are dissociated and plated at 5 × 103 cells/well in a 96-well plate. When the cells are attached to the plate, the medium is replaced with plain DMEM. The cells are treated with DWK-1339. One hour after DWK-1339 treatment, 4 μL of pre-diluted 25 μM Aβ42 is added to the media, and the cells are further incubated for 18 h. For the determination of cell viability, 15 μL of 5 mg/mL MTT is added to each well and incubated for 3 h. The formazan that formed is dissolved in DMSO, and the absorbance is measured at 570-630 nm using a plate reader[1]. |
| Animal Admin | For this study, a total of 24 (n = 8 for each group) APP/PS1 [B6C3-Tg (APPswe, PSEN1dE9) 85Dbo/J] Tg mice are utilized. The mice are housed in a controlled environment under standard room temperature, relative humidity and a 12 h light/dark cycle with free access to food and water. APP/PS1 treated groups are orally administered with DWK-1339 at a dose of 30 or 100 mg/kg body weight once daily. DWK-1339 treatment is at the age of 29 weeks, and the treatment is conducted for 8 weeks[1]. |
| References |
| Molecular Formula | C20H22O4 |
|---|---|
| Molecular Weight | 326.38600 |
| Exact Mass | 326.15200 |
| PSA | 40.83000 |
| LogP | 4.69600 |
| Storage condition | 2-8℃ |
|
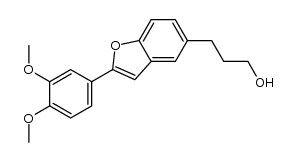
~93% 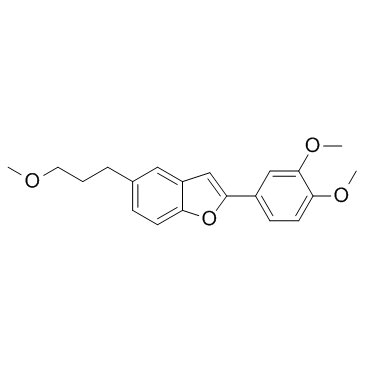
1018946-38-7 |
| Literature: DAEWOONG PHARMACEUTICAL CO., LTD.; MEDIFRON DBT CO., LTD.; CHOI, Soo Jin; LEE, Byung Goo; YOON, Hee Kyoon; LIM, Young Mook; LEE, Joon Hwan; PARK, Sung Woo Patent: WO2014/7554 A1, 2014 ; Location in patent: Paragraph 75-77 ; |
|
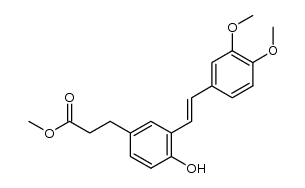
~93% 
1018946-38-7 |
| Literature: DAEWOONG PHARMACEUTICAL CO., LTD.; MEDIFRON DBT CO., LTD.; CHOI, Soo Jin; LEE, Byung Goo; YOON, Hee Kyoon; LIM, Young Mook; LEE, Joon Hwan; PARK, Sung Woo Patent: WO2014/7554 A1, 2014 ; Location in patent: Paragraph 89-91 ; |
|
~% 
1018946-38-7 |
| Literature: DAEWOONG PHARMACEUTICAL CO., LTD.; MEDIFRON DBT CO., LTD.; CHOI, Soo Jin; LEE, Byung Goo; YOON, Hee Kyoon; LIM, Young Mook; LEE, Joon Hwan; PARK, Sung Woo Patent: WO2014/7554 A1, 2014 ; Location in patent: Paragraph 98-100 ; |
|
~% 
1018946-38-7 |
| Literature: DAEWOONG PHARMACEUTICAL CO., LTD.; MEDIFRON DBT CO., LTD.; CHOI, Soo Jin; LEE, Byung Goo; YOON, Hee Kyoon; LIM, Young Mook; LEE, Joon Hwan; PARK, Sung Woo Patent: WO2014/7554 A1, 2014 ; |
|
~% 
1018946-38-7 |
| Literature: DAEWOONG PHARMACEUTICAL CO., LTD.; MEDIFRON DBT CO., LTD.; CHOI, Soo Jin; LEE, Byung Goo; YOON, Hee Kyoon; LIM, Young Mook; LEE, Joon Hwan; PARK, Sung Woo Patent: WO2014/7554 A1, 2014 ; |
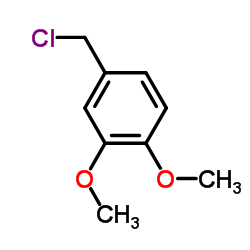
| Precursor 5 | |
|---|---|
| DownStream 0 | |