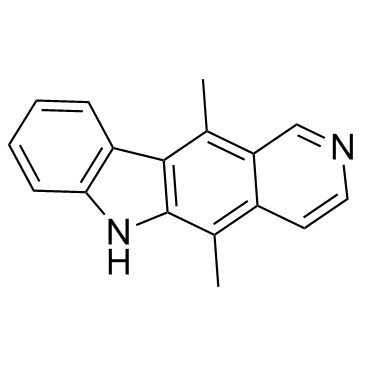
519-23-3
| Name | ellipticine |
|---|---|
| Synonyms |
MFCD00010524
5,11-Dimethyl-6H-pyrido[4,3-b]carbazole 6H-Pyrido[4,3-b]carbazole, 5,11-dimethyl- EINECS 208-264-0 tcmdc-125546 ELLIPTICINE Elliptisine |
| Description | Ellipticine hydrochloride is a potent antineoplastic agent; inhibits DNA topoisomerase II activities. |
|---|---|
| Related Catalog | |
| Target |
Topoisomerase II |
| In Vitro | Ellipticine is a potent antineoplastic agent exhibiting the multimodal mechanism of its action. The mechanisms of ellipticine antitumor, mutagenic and cytotoxic activities are suggested to be intercalation into DNA and inhibition of DNA topoisomerase II activity. Another mode of ellipticine action is the formation of covalent DNA adducts mediated by its oxidation with cytochromes P450 (CYP) and peroxidases[1]. Ellipticine can also act as an inhibitor or inducer of biotransformation enzymes, thereby modulating its own metabolism leading to its genotoxic and pharmacological effects. Treatment of cells with ellipticine results in inhibition of cell growth and proliferation. This effect is associated with formation of two covalent ellipticine-derived DNA adducts[2]. |
| In Vivo | Ellipticine treatment results in ellipticine-derived DNA adduct generation in several healthy organs (liver, kidney, lung, spleen, breast, heart and brain) and in DNA of mammary adenocarcinoma. The levels of ellipticine-derived DNA adducts generated in these adenocarcinomas are almost 2-fold higher than in normal healthy mammary tissue. The induced expression of cytochrome b5 protein in liver of rats treated with ellipticine suggests that cytochrome b5 may modulate the CYP-mediated bioactivation and detoxification of ellipticine[3]. |
| Cell Assay | The cytotoxicity of ellipticine is determined by MTT test. Ellipticine is dissolved in DMSO (1 mM) and diluted in culture medium to final concentrations of 0, 0.1, 1, 5 or 10 μM. Cells in exponential growth are seeded at 1×104 per well in a 96-well microplate. After incubation the MTT solution is added, the microplates are incubated for 4 hours and cells lysed in 50% N,N-dimethylformamide containing 20% of sodium dodecyl sulfate (SDS), pH 4.5. The absorbance at 570 nm is measured. The mean absorbance of medium controls is subtracted as a background. The viability of control cells is taken as 100% and the values of treated cells are calculated as a percentage of control. The IC50 values are calculated using linear regression of the dose-log response curves[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 495.4±40.0 °C at 760 mmHg |
| Melting Point | 316-318°C |
| Molecular Formula | C17H14N2 |
| Molecular Weight | 246.307 |
| Flash Point | 227.1±18.6 °C |
| Exact Mass | 246.115692 |
| PSA | 28.68000 |
| LogP | 4.80 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.777 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Hazard Codes | T |
|---|---|
| Risk Phrases | 25 |
| Safety Phrases | S45 |
| RIDADR | UN 3462 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | UU8825000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |