304-21-2
| Name | harmaline |
|---|---|
| Synonyms |
3,4-Dihydroharmine
Armalin 3,4-Dihydro-7-methoxy-1-methyl-9H-pyrido[3,4-b]indole 2H-Pyrido[3,4-b]indole, 3,4-dihydro-7-methoxy-1-methyl- 7-Methoxy-1-methyl-3,4-dihydro-2H-β-carboline Harmine,dihydro 7-methoxy-1-methyl-3,4-dihydro-2H-pyrido[3,4-b]indole Harmalol methyl ether EINECS 206-152-6 MFCD00004955 3,4-Dihydro-7-methoxy-1-methyl-β-carboline Dihydroharmine Harmidine O-Methylharmalol |
| Description | Harmaline is a potent and reversible monoamine oxidase inhibitor in vivo. Harmaline is a central nervous system stimulant and can be used to induce tremor in rodents. |
|---|---|
| Related Catalog | |
| Target |
Target: Monoamine oxidase[1] |
| In Vitro | Harmaline inhibits monoamine oxidases, thus increasing the levels of monoamine neurotransmitters (e.g., at 7-70 μM/kg in rats)[1]. Harmaline-induced tremor in rodents is a model of essential tremor. The tremor activity is dependent on harmaline dose. The first-line clinical essential tremor treatments propranolol, primidone and gabapentin and γ-hydroxybutyrate (GHB) significantly attenuate harmaline-induced tremor. The anticonvulsants valproate and carbamazepine and the mood stabilizer lithium suppress harmaline-induced tremor. The γ-amino-butyric acid (GABA) receptor subtype A receptor agonist muscimol attenuate harmaline-induced tremor. Harmaline (30 mg/kg) induces tremor in mice through the N-methyl-D-aspartate (NMDA) receptor, both competitive and non-competitive NMDA receptor antagonists, and AMPA receptor blockade, decreased harmaline-induced tremor[2]. |
| Animal Admin | Rats[1] Wistar albino rats of 160 to 190 g, fasted for 16 hr, receive various doses of Harmaline (0-70 μM/kg) either alone or 1 hr prior to subcutaneous administration of 75 mg/kg 5-hydroxytryptophan, 10 mg/kg Ro 4-1284 or 100 mg/kg iproniazid. The animals are decapitated and the 5-hydroxytryptamine of the brain (in the experiments with 5-hydroxytryptophan also of the heart) is measured spectrophotofluorometrically[1]. Mice[2] Group-housed mice are brought to the experimental room for at least 1 h to acclimate prior to testing. Following a 20 min pretreatment with vehicle or test compounds (60 min for primidone), drug-naive mice are injected with harmaline (30 mg/kg) and placed inside the Tremor Monitor chamber for a 10 min habituation period. Following the habituation period, tremor activity of the mice is measured for 8 min[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 426.4±45.0 °C at 760 mmHg |
| Melting Point | 232-234 °C(lit.) |
| Molecular Formula | C13H14N2O |
| Molecular Weight | 214.263 |
| Flash Point | 211.7±28.7 °C |
| Exact Mass | 214.110611 |
| PSA | 37.38000 |
| LogP | 0.66 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.647 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Risk Phrases | R25 |
| Safety Phrases | S22-S24/25 |
| RIDADR | 1544 |
| WGK Germany | 3 |
| RTECS | UU9800000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
|
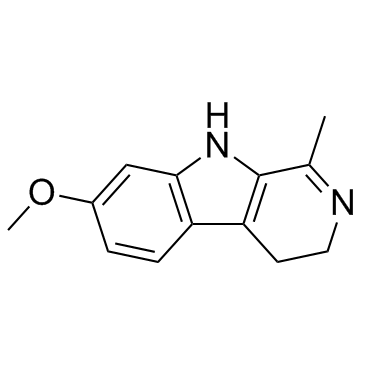
~% 
304-21-2 |
| Literature: Chemische Berichte, , vol. 63, p. 120,123 |
|
~% 
304-21-2 |
| Literature: Canadian Journal of Chemistry, , vol. 37, p. 1851,1856 |
|
~% 
304-21-2 |
| Literature: Canadian Journal of Chemistry, , vol. 37, p. 1851,1856 |
|
~% 
304-21-2 |
| Literature: Chem. Zentralbl., , vol. 72, # I p. 958 Justus Liebigs Annalen der Chemie, , vol. 64, p. 365 Chemische Berichte, , vol. 18, p. 402 Chemische Berichte, , vol. 30, p. 2482 |
|
~% 
304-21-2 |
| Literature: Chemische Berichte, , vol. 63, p. 120,123 |
| Precursor 5 | |
|---|---|
| DownStream 8 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |




![1H-Pyrrolo[2,3-c]pyridine-2,3-dicarboxylicacid, 7-methyl- structure](https://image.chemsrc.com/caspic/270/58795-15-6.png)
![2-benzyl-7-methoxy-1-methylidene-4,9-dihydro-3H-pyrido[3,4-b]indole structure](https://image.chemsrc.com/caspic/296/77784-76-0.png)
![1H-Pyrido[3,4-b]indole,2,3,4,9-tetrahydro-7-methoxy-1-methyl- (9CI) structure](https://image.chemsrc.com/caspic/429/486-93-1.png)