274693-27-5
| Name | ticagrelor |
|---|---|
| Synonyms |
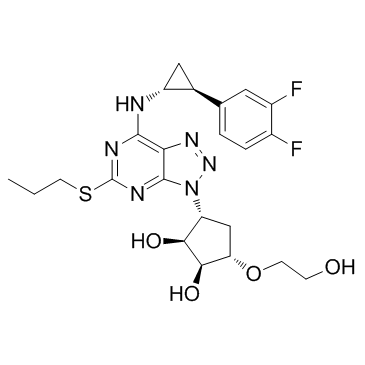
(1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino}-5-(propylsulfanyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol
Brilinta Possia 1,2-cyclopentanediol, 3-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-1,2,3-triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-, (1S,2S,3R,5S)- (1S,2S,3R,5S)-3-[7-[[(1R,2S)-2-(3,4-difluorophenyl)cyclopropyl]amino]-5-propylsulfanyltriazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)cyclopentane-1,2-diol (1S,2S,3R,5S)-3-(7-(((1R,2S)-2-(3,4-difluorophenyl)cyclopropyl)amino)-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol TICARGRELOR Ticagrelor (1S,2S,3R,5S)-3-[7-{[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino}-5-(propylsulfanyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-5-(2-hydroxyethoxy)-1,2-cyclopentanediol AZD 6140 Brilique |
| Description | Ticagrelor (AZD6140) is a reversible oral P2Y12 receptor antagonist for the treatment of platelet aggregation. |
|---|---|
| Related Catalog | |
| In Vitro | Ticagrelor promotes a greater inhibition of adenosine 5′-diphosphate (ADP)–induced Ca2+ release in ished platelets vs other P2Y12R antagonists. This additional effect of ticagrelor beyond P2Y12R antagonism is in part as a consequence of ticagrelor inhibiting the equilibrative nucleoside transporter 1 (ENT1) on platelets, leading to accumulation of extracellular adenosine and activation of Gs-coupled adenosine A2A receptors[1]. B16-F10 cells exhibit decreased interaction with platelets from ticagrelor-treated mice compared to saline-treated mice[2]. |
| In Vivo | In B16-F10 melanoma intravenous and intrasplenic metastasis models, mice treated with a clinical dose of ticagrelor (10 mg/kg) exhibits marked reductions in lung (84%) and liver (86%) metastases. Furthermore, ticagrelor treatment improves survival compared to saline-treated animals. A similar effect is observed in a 4T1 breast cancer model, with reductions in lung (55%) and bone marrow (87%) metastases following ticagrelor treatment[2]. Single oral administration of ticagrelor (1-10 mg/kg) causes dose-related inhibitory effect on platelet aggregation. Ticagrelor, at the highest dose (10 mg/kg) significantly inhibits platelet aggregation at 1 h after dosing and the peak inhibition is observed at 4 h after dosing[3]. |
| Animal Admin | Rats: Prasugrel (10 mg/kg, p.o.) and ticagrelor (30 mg/kg, p.o.), doses that produced similar levels of inhibition of platelet aggregation, are administered to rats 4 h before the bleeding time measurements. Fresh, washed platelets (1 × 1010 platelets/mL) are prepared from other rats and suspended in Hank's balanced salt solution. Platelets are transfused via the jugular vein to rats 1 h before the bleeding time measurements and the bleeding time is determined[3]. [2]Mice: Female BALB/c mice are inoculated subcutaneously in the fourth mammary pad with 4T1 breast cancer cells. Once a tumor is palpable, mice receive daily injections of PBS or ticagrelor (10 mg/kg). One week later, mice undergo primary tumor resection. At 28 days mice are sacrificed and lungs, femurs and tibiae harvested. Dissociated cells from lung and bone marrow are plated in medium containing 60 μM 6-thioguanine. After 14 days, culture plates are fixed with methanol and stained with 0.03% methylene blue to enumerate metastatic 4T1 colonies[2]. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 777.6±70.0 °C at 760 mmHg |
| Molecular Formula | C23H28F2N6O4S |
| Molecular Weight | 522.568 |
| Flash Point | 424.0±35.7 °C |
| Exact Mass | 522.186096 |
| PSA | 163.74000 |
| LogP | 1.90 |
| Vapour Pressure | 0.0±2.8 mmHg at 25°C |
| Index of Refraction | 1.744 |
| Safety Phrases | 24/25 |
|---|---|
| HS Code | 2933990090 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![(1S,2S,3R,5S)-3-(7-Chloro-5-(Propylthio)-3H-[1,2,3]Triazolo[4,5-D]Pyrimidin-3-Yl)-5-(2-Hydroxyethoxy)Cyclopentane-1,2-Diol structure](https://image.chemsrc.com/caspic/439/1354945-69-9.png)
![2-[[(3aS,4R,6S,6aR)-4-[7-[[(1R,2S)-2-(3,4-Difluorophenyl)cyclopropyl]amino]-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl]-2,2-dimethyl-tetrahydro-3aH-cyclopenta[d][1,3]dioxol-6-yl]oxy]ethanol structure](https://image.chemsrc.com/caspic/399/274693-26-4.png)
![(1S,2S,3R,5S)-3-(7-hydroxy-5-(propylthio)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-3-yl)-5-(2-hydroxyethoxy)cyclopentane-1,2-diol structure](https://image.chemsrc.com/caspic/493/1445580-43-7.png)