66803-19-8
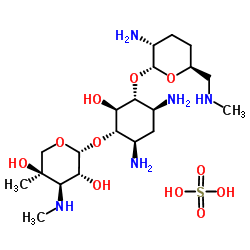
| Name | (2R,3R,4R,5R)-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,6S)-3-amino-6-(methylaminomethyl)oxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-5-methyl-4-(methylamino)oxane-3,5-diol,sulfuric acid |
|---|---|
| Synonyms |
α-D-erythro-Hexopyranoside, (1R,2S,3S,4R,6S)-4,6-diamino-3-[[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl]oxy]-2-hydroxycyclohexyl 2-amino-2,3,4,6-tetradeoxy-6-(methylamino)-, sulfate (1:1) (salt)
α-D-erythro-Hexopyranoside, (1R,2S,3S,4R,6S)-4,6-diamino-3-[[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl]oxy]-2-hydroxycyclohexyl 2-amino-2,3,4,6-tetradeoxy-6-(methylamino)-, sulfat e (1:1) (salt) (1R,2S,3S,4R,6S)-4,6-Diamino-3-{[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl]oxy}-2-hydroxycyclohexyl 2-amino-2,3,4,6-tetradeoxy-6-(methylamino)-α-D-erythro-hexopyranoside sulfate ( 1:1) (1R,2S,3S,4R,6S)-4,6-Diamino-3-{[3-deoxy-4-C-methyl-3-(methylamino)-β-L-arabinopyranosyl]oxy}-2-hydroxycyclohexyl 2-amino-2,3,4,6-tetradeoxy-6-(methylamino)-α-D-erythro-hexopyranoside sulfate (1:1) Micronomycin sulfate micronomicin sulfate Sagamacin |
| Description | Micronomicin sulfate (Gentamicin C2b sulfate) is an aminoglycoside antibiotic isolated from Micromonospora. Micronomicin sulfate is a broad-spectrum antibiotic close to the gentamicin-type antibiotics, exhibits a high activity against Pseudomonas, Proteus, Klebsiella pneumoniae, Serratia, etc (MIC=0.001-8.3 μg/ml)[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MIC: 0.001-8.3 μg/ml (Pseudomonas, Proteus, Klebsiella pneumoniae, Serratia)[2] |
| In Vitro | Micronomicin has a potent antibacterial activity, it is active against Staphylococcus aureus FDA 209 P, Staphylococcus aureus with the minimal inhibitory values of 0.01 μg/ml. It is also against Escherichia coli St.M. 589, Baker 2, F 14-BK, and R5/W677 with the minimal inhibitory values of 0.75 μg/ml, 0.3 μg/ml, 0.03 μg/ml and 0.03 μg/ml. And it is active against Pseudomonas aeruginosa strains and lebsiella pneumoniae strains (MICs = 0.03-17.5 μg/ml)[1]. |
| In Vivo | Micronomicin sulfate is highly active against various bacterial infections in mice, and has an intravenous acute LD50 in mice of 93 mg/kg[1]. Micronomicin sulfate (intravenous injection; 4-100 mg/kg; 30 days) is injected for subacute toxicity study. The wistar rats dies at the dose level of 100 mg/kg (10 out of 30 animals): renal disorders and ataxia. The renal histological disorders occurrs mainly at the dose levels of 25 mg/kg and over[3]. Animal Model: Wistar rats[3] Dosage: 4, 10, 25, 63 mg/kg and 100 mg/kg Administration: Intravenous injection; 30 days Result: Led to death of rat at 100 mg/kg. |
| References |
| Density | 1.32g/cm3 |
|---|---|
| Boiling Point | 667.2ºC at 760mmHg |
| Molecular Formula | C20H41N5O7.xH2O4S |
| Molecular Weight | 561.647 |
| Flash Point | 357.3ºC |
| Exact Mass | 561.268005 |
| PSA | 282.71000 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|