70030-11-4
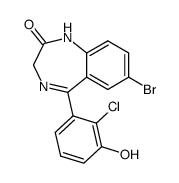
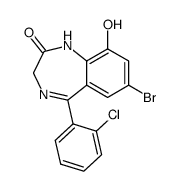
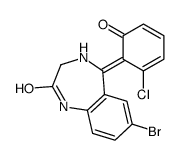
| Name | 7-bromo-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-1,4-benzodiazepin-2-one |
|---|---|
| Synonyms |
3-Hydroxyfenazepam
7-bromo-5-(2-chlorophenyl)-3-hydroxy-1,2-dihydro-3H-1,4-benzodiazepin-2-one 3-Oxyfenazepam 7-bromo-5-(2-chloro-phenyl)-3-hydroxy-1,3-dihydro-benzo[e][1,4]diazepin-2-one 7-Bromo-5-(2-chlorophenyl)-3-hydroxy-1,3-dihydro-2H-1,4-benzodiazepin-2-one 3-Hydroxyphenazepam 2H-1,4-Benzodiazepin-2-one, 7-bromo-5-(2-chlorophenyl)-1,3-dihydro-3-hydroxy- |
| Description | 3-Hydroxyphenazepam is an active metabolite of Cinazepam. Cinazepam is a GABAA receptor agonist. 3-Hydroxyphenazepam can inhibit synaptosomal transporter-mediated [3H]GABA uptake[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 544.5±50.0 °C at 760 mmHg |
| Molecular Formula | C15H10BrClN2O2 |
| Molecular Weight | 365.61 |
| Flash Point | 283.1±30.1 °C |
| Exact Mass | 363.961395 |
| PSA | 65.18000 |
| LogP | 2.46 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.712 |
|
~38% 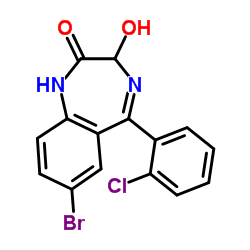
70030-11-4 |
| Literature: Davidenko, T. I.; Zabolotskaya, N. N.; Milienko, N. P.; Andronati, S. A.; Kuznetsov, V. D.; Bogatskii, A. V. Doklady Chemistry, 1984 , vol. 278, p. 335 - 337 Dokl. Akad. Nauk SSSR Ser. Khim., 1984 , vol. 278, # 4 p. 878 - 881 |
|
~%
Detail
|
| Literature: Golovenko, N. Ya.; Zin'kovskii, V. G.; Bogatskii, A. V.; Sharbatyan, P. A.; Andronati, S. A. Pharmaceutical Chemistry Journal, 1980 , vol. 14, # 4 p. 208 - 213 Khimiko-Farmatsevticheskii Zhurnal, 1980 , vol. 14, # 4 p. 15 - 21 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |