20874-31-1
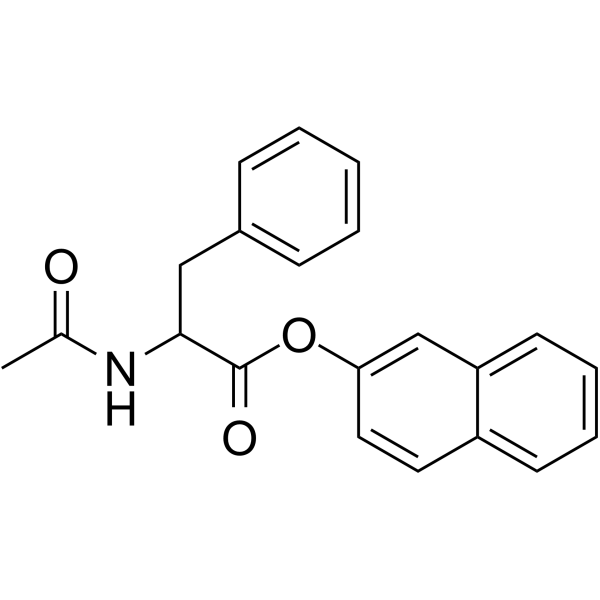
| Name | β-naphthyl N-acetylphenylalaninate |
|---|---|
| Synonyms |
beta-naphthyl N-acetylphenylalaninate
APNE N-Acetyl-DL-phenylalanin-[2]naphthylester N-acetyl-DL-phenylalanine-[2]naphthyl ester naphthalen-2-yl 2-acetamido-3-phenylpropanoate mv1 |
| Description | N-Acetyl-DL-phenylalanine β-naphthyl ester is an aromatic amino acid ester, which functions as a chromogenic substrate for chymotrypsin and microbial serine proteases such as subtilisin[1]. |
|---|---|
| Related Catalog | |
| In Vitro | N-Acetyl-DL-phenylalanine β-naphthyl ester is hydrolyzed in the body by the esterase (APNEase) catalytic action at neutral pHs. APNEase level involves with many muscle wasting conditions suggest the possibility that it may be involved in the turnover and pathological breakdown of muscle proteins[1]. N-Acetyl-DL-phenylalanine β-naphthyl ester (0.75 mM, DMF; 3 min) as substrate and o-Dianisidine tetrazotized (oD) as the dye, allow the assessment of protease inhibitory activity directly from the yeast P. pastoris expression media[2]. N-Acetyl-DL-phenylalanine β-naphthyl ester (0.75 mM, DMF; 3 min) (2.4 g/L) provides a visualization result with stained agar gel via the diazo coupling of the β-naphtol produced by the enzymatic hydrolysis of N-Acetyl-DL-phenylalanine β-naphthyl ester (APNE). Circular zones containing inhibitor-proteinase complexes remain coloress while the region containing only proteinase shows a bright pink-purple color[3]. N-Acetyl-DL-phenylalanine β-naphthyl ester (5 mg/40 mL) has application: determine the class of peptidase in mouse plasma. The enzyme was displayed by immunoprecipitation with antiserum in radial immunodiffusion. After removal of non-precipitated serum and other constituents by washing in excess saline, individual rings of immunoprecipitate were incubated in a solution of a protease inhibitor, followed by washing and staining with the chromogenic substrate (NAPBNE and fast blue B), the picture can be photographed over direct lighting[4]. |
| References |
| Density | 1.2g/cm3 |
|---|---|
| Boiling Point | 589.8ºC at 760mmHg |
| Molecular Formula | C21H19NO3 |
| Molecular Weight | 333.38000 |
| Flash Point | 310.5ºC |
| Exact Mass | 333.13600 |
| PSA | 55.40000 |
| LogP | 3.88350 |
| Vapour Pressure | 6.93E-14mmHg at 25°C |
| Index of Refraction | 1.618 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
~% 
20874-31-1 |
| Literature: Journal of the American Chemical Society, , vol. 73, p. 2086,2088 |