502632-66-8
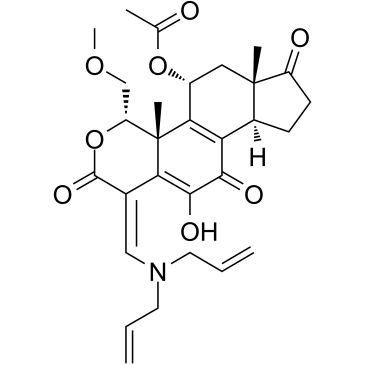
| Name | [(3aR,6E,9S,9aR,10R,11aS)-6-[[bis(prop-2-enyl)amino]methylidene]-5-hydroxy-9-(methoxymethyl)-9a,11a-dimethyl-1,4,7-trioxo-2,3,3a,9,10,11-hexahydroindeno[4,5-h]isochromen-10-yl] acetate |
|---|---|
| Synonyms |
Cyclopenta[5,6]naphtho[1,2-c]pyran-2,7,10(1H)-trione, 5-(acetyloxy)-1-[(di-2-propen-1-ylamino)methylene]-4,4a,5,6,6a,8,9,9a-octahydro-11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-, (1E,4S,4aR,5R,6aS,9aR)-
PX-866 (1E,4S,4aR,5R,6aS,9aR)-1-[(diprop-2-en-1-ylamino)methylidene]-11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-2,7,10-trioxo-1,2,4,4a,5,6,6a,7,8,9,9a,10-dodecahydroindeno[4,5-h]isochromen-5-yl acetate DJM-2-166 (1E,4S,4aR,5R,6aS,9aR)-1-[(Diallylamino)methylene]-11-hydroxy-4-(methoxymethyl)-4a,6a-dimethyl-2,7,10-trioxo-1,2,4,4a,5,6,6a,7,8,9,9a,10-dodecahydroindeno[4,5-h]isochromen-5-yl acetate DJM-166 Sonolisib |
| Description | Sonolisib (PX-866), an improved Wortmannin analogue, is an oral, irreversible, and pan-isoform inhibitor of PI3K (IC50=0.1 nM (p110α), 1.0 nM (p120γ), 2.9 nM (p110δ)). Antitumor activity[1][2]. |
|---|---|
| Related Catalog | |
| Target |
p110α:0.1 nM (IC50) p110δ:2.9 nM (IC50) p120γ:1 nM (IC50) |
| In Vitro | Sonolisib (PX-866) inhibits the production of the secondary messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) and activation of the PI3K/Akt signaling pathway, which may result in inhibition of tumor cell growth and survival in susceptible tumor cell populations[3]. |
| References |
[3]. Sonolisib |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 651.4±55.0 °C at 760 mmHg |
| Molecular Formula | C29H35NO8 |
| Molecular Weight | 525.590 |
| Flash Point | 347.7±31.5 °C |
| Exact Mass | 525.236267 |
| PSA | 119.44000 |
| LogP | 2.37 |
| Vapour Pressure | 0.0±4.4 mmHg at 25°C |
| Index of Refraction | 1.586 |