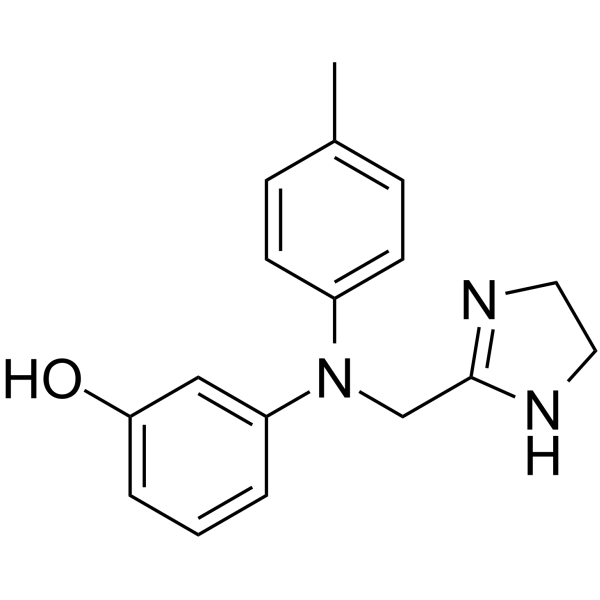
50-60-2
| Name | phentolamine |
|---|---|
| Synonyms |
Fentolamin
EINECS 200-053-1 MFCD00242985 Phenol, 3-(((4,5-dihydro-1H-imidazol-2-yl)methyl)(4-methylphenyl)amino)- 2-(N-[m-Hydroxyphenyl]-p-toluidinomethyl)imidazoline Phenol, 3-[[(4,5-dihydro-1H-imidazol-2-yl)methyl](4-methylphenyl)amino]- Phentalamine Regitin Phenotolamine 3-[(4,5-Dihydro-1H-imidazol-2-ylmethyl)(4-methylphenyl)amino]phenol Dibasin Fentolamina Regitine Phentolaminum Phentolamine 3-[N-(4,5-dihydro-1H-imidazol-2-ylmethyl)-4-methylanilino]phenol Rogitine |
| Description | Phentolamine is a potent, selective and orally active α1 adrenergic and α2 adrenergic receptor antagonist. Phentolamine can be used for the treatment of erectile dysfunction[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vivo | Phentolamine (5-20 mg/kg; i.p.) effectively inhibits the seizures elicited by strychnine (2 mg/kg, i.p.) and attenuates the seizure-potentiating effect of DOPS (4 mg/kg, i.p.) in mouse[2]. Phentolamine (1 mg/kg; i.p.) increases insulin secretion by inhibition of b-cell a2A-adrenoceptors in mouse[3]. Animal Model: WT mice[3] Dosage: 1 mg/kg Administration: IP Result: Reduced blood glucose and increased insulin levels. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 551.0±45.0 °C at 760 mmHg |
| Melting Point | 177 - 178ºC |
| Molecular Formula | C17H19N3O |
| Molecular Weight | 281.352 |
| Flash Point | 287.0±28.7 °C |
| Exact Mass | 281.152802 |
| PSA | 47.86000 |
| LogP | 3.60 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.626 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Safety Phrases | S26-S36/37/39-S45-S8-S25 |
|---|---|
| RIDADR | UN 3264 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 2933290090 |
|
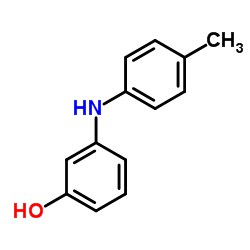
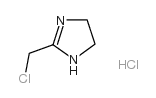
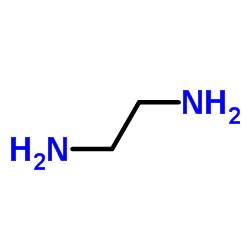
~% 
50-60-2 |
| Literature: CIBA Patent: DE842062 , 1948 ; DRP/DRBP Org.Chem. Full Text Show Details CIBA Patent: US2503059 , 1948 ; Full Text Show Details Urech et al. Helvetica Chimica Acta, 1950 , vol. 33, p. 1386,1403 |
|
~% 
50-60-2 |
| Literature: CIBA Patent: DE842062 , 1948 ; DRP/DRBP Org.Chem. Full Text Show Details CIBA Patent: US2503059 , 1948 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |