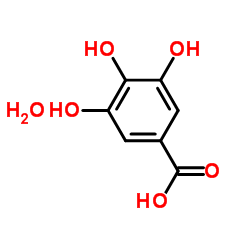
5995-86-8
| Name | Gallic acid monohydrate |
|---|---|
| Synonyms |
Benzoic acid, 3,4,5-trihydroxy-, hydrate (1:1)
Gallic acid monohydrate 3,4,5-Trihydroxybenzoic acid hydrate (1:1) MFCD00002510 3,4,5-trihydroxybenzoic acid,hydrate EINECS 205-749-9 |
| Description | Gallic acid (3,4,5-Trihydroxybenzoic acid) hydrate is a natural polyhydroxyphenolic compound and an free radical scavenger to inhibit cyclooxygenase-2 (COX-2)[1]. Gallic acid hydrate has various activities, such as antimicrobial, antioxidant, antimicrobial, anti-inflammatory, and anticance activities[2]. |
|---|---|
| Related Catalog | |
| Target |
COX-2 Human Endogenous Metabolite |
| In Vitro | Gallic acid is an antioxidant which can inhibit both COX-2[1]. After 18 h treatment with Gallic acid, the number of viable neutrophils is dramatically decreased from 40.3% to 27.7%, highly comparable with 26.4% for untreated neutrophils. Gallic acid fails to attenuate isoproterenol-induced myocytolysis[3]. |
| In Vivo | The food intake (2.6±0.08 g/day, p=0.69) and the body weight (2.5±0.69 g, p=0.76) of the Gallic acid group do not differ significantly from those of the control group (food intake; 2.41±0.14 g/day and the body weight; 2.83±0.84 g/day). The blood glucose tolerance in the Gallic acid group is significantly improved after 2 weeks of treatment. The blood glucose tolerance of the Gallic acid group after a treatment period of 2 weeks is also significantly better than that of the control group at 90 and 120 min ( p<0.05). The serum triglyceride concentration in the Gallic acid group (0.67±0.03 mM, p<0.05) is significantly reduced relative to that of the control group (1.08±0.20 mM). The total cholesterol concentration is similar in the control (3.19±0.27 mM) and Gallic acid (3.01±0.18 mM) groups[2]. |
| References |
| Density | 1.694 |
|---|---|
| Boiling Point | 596.6ºC at 760 mmHg |
| Melting Point | 252 °C (dec.)(lit.) |
| Molecular Formula | C7H8O6 |
| Molecular Weight | 188.135 |
| Flash Point | 250 °C |
| Exact Mass | 188.032089 |
| PSA | 107.22000 |
| LogP | 0.43730 |
| Water Solubility | 15 g/l (20 ºC) |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | LW7525000 |
| HS Code | 2918290000 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2918290000 |
|---|---|
| Summary | HS: 2918290000 other carboxylic acids with phenol function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) VAT:17.0% MFN tariff:6.5% General tariff:30.0% |