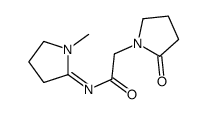
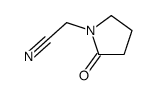
7491-74-9
| Name | 2-(2-oxopyrrolidin-1-yl)acetamide |
|---|---|
| Synonyms |
2-pyrrolidoneacetamide
Pyracetam 2-(2-Oxo-1-pyrrolidinyl)acetamide Gabacet Normabrain Nootropil Nootrop Ciclofalina 2-Oxo-1-pyrrolidineacetamide 1-Pyrrolidineacetamide, 2-oxo- 2-(2-oxopyrrolidin-1-yl)acetamide 5-21-06-00360 (Beilstein Handbook Reference) MFCD00079246 Piracetam Nootropyl Pyramem EINECS 231-312-7 Oxiracetam Impurity 1 |
| Description | Piracetam is a cyclic derivative of the neurotransmitter gamma-aminobutyric acid (GABA), used in treatment of a wide range of cognitive disorders.Target: OthersPiracetam is able to significantly decrease the fusogenic and destabilising effect of Abeta 29-42, in a concentration-dependent manner. Preincubation of piracetam, at a piracetam/peptide ratio of 960, during 20 min before the addition of Abeta 29-42 prevents almost completely the mixture of the two fluorescent probes. Preincubation of piracetam with lipids prevents almost completely the release of calcein induced by the peptide in a dose-dependent fashion (piracetam/peptide ratios from 9.6 to 960) [1]. Piracetam (< 1.0 mM) preincubated with brain membranes enhances membrane fluidity in aged mice, rats and humans, as indicated by decreased anisotropy of the membrane-bound fluorescence probe 1,6-diphenyl-1,3,5-hexatriene (DPH). Piracetam (300 mg/kg once daily) significantly increases membrane fluidity in some brain regions of young and aged rats, but has no measurable effect on membrane fluidity in the young rats [2]. Piracetam (300 mg/kg daily for 6 weeks) improves active avoidance learning in the aged rats only and elevates membrane fluidity in all brain regions except the cerebellum in the aged rats. Piracetam (300 mg/kg daily for 6 weeks) also improves NMDA receptor density in the hippocampus and on muscarinic cholinergic receptor densities in the frontal cortex and the striatum and to a lesser extent in the hippocampus of rats [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 337.3±44.0 °C at 760 mmHg |
| Melting Point | 151-152ºC |
| Molecular Formula | C6H10N2O2 |
| Molecular Weight | 142.156 |
| Flash Point | 157.8±28.4 °C |
| Exact Mass | 142.074234 |
| PSA | 63.40000 |
| LogP | -1.39 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.603 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | UX9660500 |
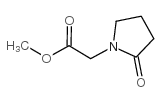
| Precursor 9 | |
|---|---|
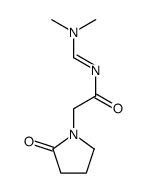
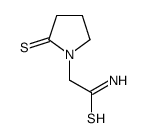
| DownStream 6 | |

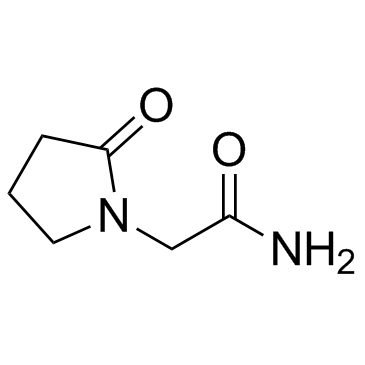
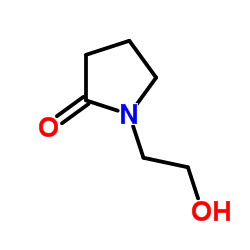
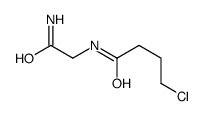
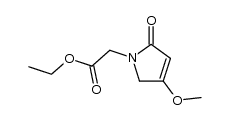
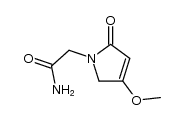
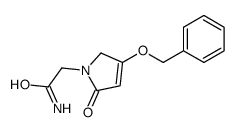

![2-[(2E)-2-(nitromethylidene)pyrrolidin-1-yl]acetonitrile structure](https://image.chemsrc.com/caspic/490/91417-82-2.png)