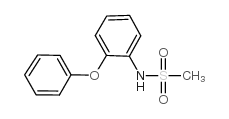
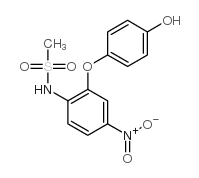
51803-78-2
| Name | nimesulide |
|---|---|
| Synonyms |
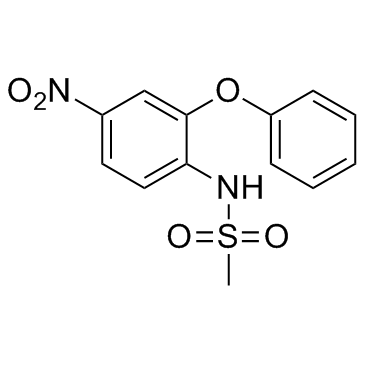
N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide
N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide Nimesulide EINECS 257-431-4 AULIN N-(2-phenoxy-4-nitrophenyl)methanesulfonamide Nimepast Mesulid Flogovital Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)- Sulidene Antifloxil Nimed 4-nitro-2-phenoxymethanesulfonanilide Nisulid Guaxan R-805 Nimesulide N-(4-Nitro-2-phenoxy-phenyl)-methanesulfonamide MFCD00079470 |
| Description | Nimesulide is a selective COX-2 inhibitor, with IC50s of 70 nM-70 μM in a time-dependent manner, but it shows no effect on COX-1 (IC50 >100 μM). Nimesulide has potent anti-inflammatory, analgesic and antipyretic properties. |
|---|---|
| Related Catalog | |
| Target |
COX-2:0.07-70 μM (IC50) |
| In Vitro | Nimesulide is a selective COX-2 inhibitor, with IC50s of 70 nM-70 μM in a time-dependent manner, but it shows weak effect on COX-1 (IC50 >100 μM)[1]. Nimesulide (10 µM) effectively decreases VEGF in endometrium cancer cells, and shows no effect on that in normal cells. Nimesulide (10 and 50 µM) dramatically decreases MCP-1 levels in normal cell, and such an effect is also observed with 10 µM in cancer cells. In addition, Nimesulide (50 µM) potently affects IL-8 level in normal cells, but causes no changes in cancer cells[3]. |
| In Vivo | Nimesulide (3 and 10 mg/kg, i.p.) effectively blocks fever induced by i.p. injection of LPS in rats. Nimesulide (3 mg/kg, i.p.) potently reduces fever response induced by IL-1β, IL-6 or TNF-α, but does not prevent the initial rise in the febrile response induced by arachidonic acid. Nimesulide also significantly reduces PGE2 levels and PGF2α levels in the cerebrospinal fluid of the LPS-stimulated animals, and inhibits the increase in plasma TNF-α by 97%[2]. |
| Cell Assay | 5×105 viable cells are carefully dislodged with sterile Pasteur pipettes, transferred into new flasks and incubated with two different doses of Nimesulide (10 and 50 µM) for another 24 h. Incubations for different doses of Nimesulide are repeated three times. The culture supernatant is then collected and stored in small aliquots at -70°C until studied. VEGF, MCP-1 and IL-8 concentrations are determined by sandwich quantitative enzyme immunoassay (ELISA) using commercial kits[3]. |
| Animal Admin | Rats[2] In the initial experiments, rats are pre-treated with intraperitoneal injections of 1, 3 or 10 mg/kg doses of Nimesulide, diluted in a 5% cremophor vehicle, or 2 mg/kg of indomethacin diluted in tris(hydroximetyl)-aminomethane-HCl (TRIS), pH 8.2, 30 min prior to an i.p. injection of LPS (50 μg/kg). Control animals receive the appropriate vehicle plus saline (1 mL/200 g, i.p.). The dose of 3 mg/kg of Nimesulide is chosen for the remaining experiments. In another set of experiments, rats are pretreated with an i.p. injection of Nimesulide (3 mg/kg) or indomethacin (2 mg/kg), diluted in the appropriate vehicles, 30 min prior to an i.c.v. injection (2 μL over 1 min) of IL-1β (3.12 ng), IL-6 (300 ng), TNF-α (250 ng), arachidonic acid (50 μg), MIP-1α (500 ng), PGE2 (250 ng), PGF2α (250 ng), CRF (2 μg) or ET-1 (1 pmol). Control animals receive the appropriate vehicles (1 mL/200 g, i.p.) and sterile saline (2 μL over 1 min, i.c.v.). All the drugs are injected between 10:00 and 11:00 AM to avoid circadian rhythm variations[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 442.0±55.0 °C at 760 mmHg |
| Melting Point | 140-146°C |
| Molecular Formula | C13H12N2O5S |
| Molecular Weight | 308.310 |
| Flash Point | 221.1±31.5 °C |
| Exact Mass | 308.046692 |
| PSA | 109.60000 |
| LogP | 3.79 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.638 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn: Harmful;Xi: Irritant; |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | PB0970000 |
| Packaging Group | III |
|
~% 
51803-78-2 |
| Literature: Lunghi, Angelo; Alos, Miquel A.; Gigante, Lucia; Feixas, Joan; Sironi, Eugenio; Feliu, Josep A.; Cardillo, Paolo Organic Process Research and Development, 2002 , vol. 6, # 6 p. 926 - 932 |
| Precursor 1 | |
|---|---|
| DownStream 7 | |