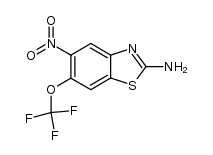
1744-22-5
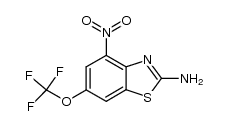
| Name | 2-Amino-6-(trifluoromethoxy)benzo[d]thiazole |
|---|---|
| Synonyms |
MFCD00210213
2-Benzothiazolamine, 6-(trifluoromethoxy)- 2-Amino-6-(trifluoromethoxy)benzothiazole 6-(Trifluoromethoxy)-1,3-benzothiazol-2-amine Riluzole 2-Amino-6-trifluoro methoxy benzothiazole 2-Amino-6-trifluoromethoxybenzothiazole 6-(Trifluoromethoxy)-2-benzothiazolamine Rilutek 6-(Trifluoromethoxy)benzo[d]thiazol-2-amine |
| Description | Riluzole is an anticonvulsant drug and belongs to the family of use-dependent Na+ channel blocker which can also inhibit GABA uptake with an IC50 of 43 μM. |
|---|---|
| Related Catalog | |
| Target |
Sodium channel[1] IC50: 43 μM (GABA receptor)[1] |
| In Vitro | Riluzole is an anticonvulsant drug and belongs to the family of use-dependent Na+ channel blocker which can also inhibit GABA uptake with an IC50 of 43 μM. At 20 μM, Riluzole inhibits peak autaptic IPSCs only slightly but prolongs IPSCs reliably. It is also found that Riluzole causes a strong, concentration-dependent, readily reversible enhancement of responses to 2 μM GABA. At higher concentrations of Riluzole, especially 300 μM, GABA currents exhibit apparent desensitization during prolonged co-exposure to 2 μM GABA and Riluzole. The EC50 of Riluzole potentiation of GABA responses is about 60 μM[1]. |
| In Vivo | In normal naïve rats, systemic injection of Riluzole (8 mg/kg, i.p.; n=6 rats) decreases the duration of ultrasonic but not audible vocalizations evoked by noxious stimulation of the knee joint compare to vehicle tested in the same rats (P<0.05). Systemic application of Riluzole (8 mg/kg, i.p.; n=19 rats) decreases the vocalizations of arthritic rats compare to predrug and vehicle significantly (P<0.05 to 0.001). Riluzole administered into the CeA significantly decreases the duration of audible and ultrasonic vocalizations evoked by noxious stimulation of the knee compare to predrug values (n=8 rats; P<0.05 to 0.01)[2]. |
| Cell Assay | Two-electrode voltage clamp of Xenopus oocytes expressing exogenous GABAA receptors is performed with a CA-1B high performance oocyte clamp. The extracellular recording solution is ND-96 medium. Riluzole is applied from a common tip via a gravity-driven multibarrel drug-delivery system. Data acquisition and analysis are performed with pCLAMP 6 software[1]. |
| Animal Admin | Adult male Sprague-Dawley rats (180 to 350 g) are housed in a temperature-controlled room and maintained on a 12-h day/night cycle with unrestricted access to food and water. Pain behaviors are measured before and 5 h after induction of a mono-arthritis in the left knee joint. To test the effects of systemic (intraperitoneal, i.p.) application of Riluzole, pain behaviors are measured 1 h postinjection of Riluzole in normal and arthritic animals. To determine effects of Riluzole into the amygdala, pain behaviors are measured 15 min after starting Riluzole application through a stereotaxically implanted microdialysis probe. To investigate site of action in the amygdala of systemically applied Riluzole, potassium channel blockers are administered into the amygdala 45 min after systemic application of Riluzole and pain behaviors are measured 15 min later, i.e., 1 h postinjection of riluzole (i.p.)[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 296.3±50.0 °C at 760 mmHg |
| Melting Point | 116-118ºC |
| Molecular Formula | C8H5F3N2OS |
| Molecular Weight | 234.198 |
| Flash Point | 133.0±30.1 °C |
| Exact Mass | 234.007462 |
| PSA | 76.38000 |
| LogP | 2.84 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.615 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ≥25 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 |
| Precautionary Statements | P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| RTECS | DL2830000 |
| Packaging Group | II |
| HS Code | 2934999090 |
|
~78% 
1744-22-5 |
| Literature: Anzini, Maurizio; Chelini, Alessia; Mancini, Alessandra; Cappelli, Andrea; Frosini, Maria; Ricci, Lorenzo; Valoti, Massimo; Magistretti, Jacopo; Castelli, Loretta; Giordani, Antonio; Makovec, Francesco; Vomero, Salvatore Journal of Medicinal Chemistry, 2010 , vol. 53, # 2 p. 734 - 744 |
|
~87% 
1744-22-5 |
| Literature: BRISTOL-MYERS SQUIBB COMPANY; UNIVERSITE DE MONTREAL; LAWRENCE, Michael, R.; MILLER, Michael, M.; SEIFFERT, Dietmar, Alfred; POSY, Shoshana, L.; WONG, Pancras, C.; BANVILLE, Jacques; RUEDIGER, Edward, H.; DEON, Daniel, H.; MARTEL, Alain; TREMBLAY, Francois; GUY, Julia; LAVALLEE, Jean-Francois; GAGNON, Marc Patent: WO2013/163244 A1, 2013 ; Location in patent: Paragraph 00205; 00206 ; |
|
~73% 
1744-22-5 |
| Literature: Jordan, Alfonzo D.; Luo, Chi; Reitz, Allen B. Journal of Organic Chemistry, 2003 , vol. 68, # 22 p. 8693 - 8696 |
|
~% 
1744-22-5 |
| Literature: US5236940 A1, ; |
|
~% 
1744-22-5 |
| Literature: US5068238 A1, ; |
| Precursor 6 | |
|---|---|
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |