60142-96-3
| Name | Gabapentin |
|---|---|
| Synonyms |
Gabapetin
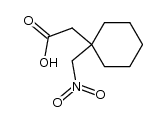
Neurontin 1-(Aminomethyl)-cyclohexaneacetic acid 2-[1-(aminomethyl)cyclohexyl]acetic acid Cyclohexaneacetic acid, 1-(aminomethyl)- GABAPENTINE GABAPENTIN Gabapentin hydrochloride 2-[(aminomethyl)cyclohexyl]acetic acid 1-(aminomethyl)cyclohexaneacetic acid MFCD00865286 2-(1-(aminomethyl)cyclohexyl)acetic acid EINECS 262-076-3 Gababentin [1-(Aminomethyl)cyclohexyl]acetic acid Gabapentin HCl |
| Description | Gabapentin (Neurontin) is a pharmaceutical drug, specifically a GABA analog. It was originally developed to treat epilepsy, and currently is also used to relieve neuropathic pain.IC50 Value: 140 nM (α2δ subunit of calcium channel) [1]Target: Calcium Channelin vitro: Gabapentin, baclofen and CGP 44532 all reduced the electrically stimulated release of [3H]glutamic acid (IC50=20 microM, 0.8 microM and 2 microM, respectively). Gabapentin was without effect on the release of [3H]GABA, whilst baclofen (IC50=8 microM) and CGP 44532 (IC50=1 microM) inhibited [3H]GABA release [2]. A large inhibition of calcium currents by gabapentin was observed in pyramidal neocortical cells (up to 34%). Significantly, the gabapentin-mediated inhibition of calcium currents saturated at particularly low concentrations (around 10 microM), at least in neocortical neurons (IC50 about 4 microM) [3].in vivo: Gabapentin produced an anti-allodynic effect over the 7-day period, reducing the expression of pro-inflammatory cytokines but increasing the expression of IL-10 (TNF-α, 316.0 ± 69.7 pg/mL vs 88.8 ± 24.4 pg/mL; IL-1β, 1,212.9 ± 104.5 vs 577.4 ± 97.1 pg/mL; IL-6, 254.0 ± 64.8 pg/mL vs 125.5 ± 44.1 pg/mL; IL-10, 532.1 ± 78.7 pg/mL vs 918.9 ± 63.1 pg/mL). The suppressive effect of gabapentin on pro-inflammatory cytokine expression was partially blocked by the anti-IL-10 antibody [4].Toxicity: No new safety signals or adverse event trends relating to GEn exposure were identified [5].Clinical trial: N/A |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 314.4±15.0 °C at 760 mmHg |
| Melting Point | 162°C |
| Molecular Formula | C9H17NO2 |
| Molecular Weight | 171.237 |
| Flash Point | 144.0±20.4 °C |
| Exact Mass | 171.125931 |
| PSA | 63.32000 |
| LogP | 1.19 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.489 |
| Storage condition | Desiccate at +4°C |
| Water Solubility | H2O: 10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H319-H335-H360 |
| Precautionary Statements | P201-P261-P305 + P351 + P338-P308 + P313 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Phrases | R61 |
| Safety Phrases | S53-S26-S36/37/39-S45 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | GU6496000 |
| HS Code | 2922499990 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2922499990 |
|---|---|
| Summary | HS:2922499990 other amino-acids, other than those containing more than one kind of oxygen function, and their esters; salts thereof VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |

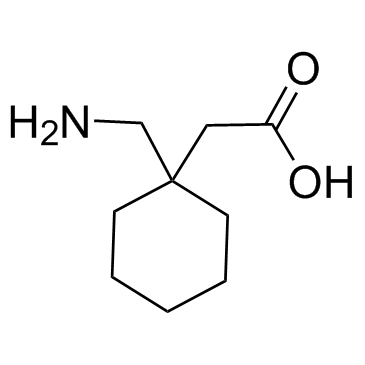
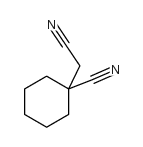
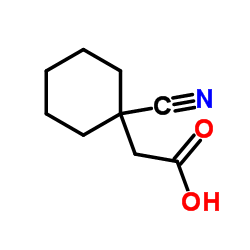
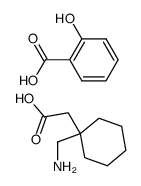

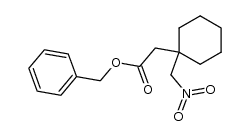

![2-[1-(aminomethyl)cyclohexyl]acetic acid,sulfuric acid structure](https://image.chemsrc.com/caspic/042/585540-04-1.png)