144689-63-4
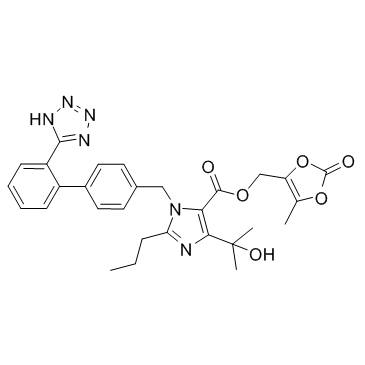
| Name | Olmesartan medoxomil |
|---|---|
| Synonyms |
Benicar
MFCD00914967 (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl-4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazol-5-carboxylat 1H-imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(2H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxy-2-propanyl)-2-propyl-1-{[2'-(1H-tetrazol-5-yl)-4-biphenylyl]methyl}-1H-imidazole-5-carboxylate (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(1H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazole-5-carboxylate (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazole-5-carboxylate 4-(1-hydroxy-1-méthyléthyl)-2-propyl-1-{[2'-(2H-tétrazol-5-yl)biphényl-4-yl]méthyl}-1H-imidazole-5-carboxylate de (5-méthyl-2-oxo-1,3-dioxol-4-yl)méthyle Olmetec (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-{[2'-(2H-tetrazol-5-yl)biphenyl-4-yl]methyl}-1H-imidazole-5-carboxylate 1H-Imidazole-5-carboxylic acid, 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-yl]methyl]-, (5-methyl-2-oxo-1,3-dioxol-4-yl)methyl ester Benevas Olmesartan Medoxomil |
| Description | Olmesartan medoxomil is a potent and selective angiotensin AT1 receptor inhibitor with IC50 of 66.2 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 66.2 μM (angiotensin II receptor)[1] |
| In Vitro | Inhibition of Arachidonic acid (AA) metabolism by angiotensin II receptor blockers (ARBs) is detected in a concentration-dependent manner with IC50 of Olmesartan (66.2 μM)[1]. Olmesartan medoxomil (OLM) is a potent and selective angiotensin AT1 receptor blocker[2]. |
| In Vivo | The efficacy of Olmesartan (20 mg/kg) studied in db/db diabetic mice for a period of 12 weeks starting from week 10 to 12 of age. The db/db mice have 11.7 fold increased albuminuria in comparison to control mice at week 22 to 24 of age. Twelve weeks Olmesartan administration significantly reduces albuminuria in db/db mice by 77% as compared with placebo treated db/db mice. The albumin/creatinine ratio (ACR) is increased in db/db mice in comparison to control mice by 7.1 fold and Olmesartan treatment significantly decreases ACR by 59% in db/db mice[3]. |
| Animal Admin | Mice[3] 10 to 12-week old male db/db diabetic mice with background strain C57BL/KsJ and their age-matched non-diabetic lean control mice (C57BL) are used.10 non-diabetic control mice and 10 diabetic mice are fed with placebo (0.5% sodium CMC/saline solution), and 10 diabetic mice are fed with 20 mg/kg Olmesartan (MB5704) by daily gavage for 12 weeks. Mice are monitored for blood glucose, body weight and urine output every two weeks. After treatment, mice are euthanized and trunk blood is collected and is centrifuged to obtain plasma which is aliquoted and stored at -80°C. Kidney tissues are removed from mice. For protein extraction slices of the kidney tissue are frozen in liquid nitrogen, and stored at -80°C. Other parts of the kidney tissue are fixed with 4% paraformaldehyde and embedded in paraffin for immunostaining. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 804.2±75.0 °C at 760 mmHg |
| Melting Point | 180°C |
| Molecular Formula | C29H30N6O6 |
| Molecular Weight | 558.585 |
| Flash Point | 440.2±37.1 °C |
| Exact Mass | 558.222656 |
| PSA | 162.16000 |
| LogP | 5.23 |
| Vapour Pressure | 0.0±3.0 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Storage condition | -20°C Freezer |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/38:Irritating to eyes and skin . R41:Risk of serious damage to eyes. R38:Irritating to the skin. |
| Safety Phrases | S37/39-S26-S39 |
| RIDADR | NONH for all modes of transport |
| RTECS | NI4014200 |
| HS Code | 2934999090 |
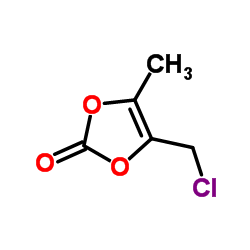
| Precursor 10 | |
|---|---|
| DownStream 1 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |


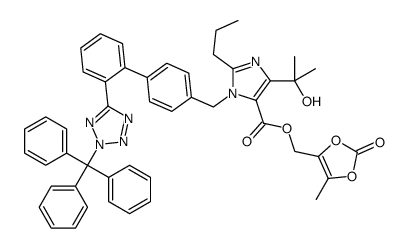
![Ethyl 4-(2-hydroxypropan-2-yl)-2-propyl-1-((2'-(1-trityl-1H-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl)methyl)-1H-imidazole-5-carboxylate structure](https://image.chemsrc.com/caspic/229/144690-33-5.png)
![5-(4'-(Bromomethyl)-[1,1'-biphenyl]-2-yl)-1-trityl-1H-tetrazole structure](https://image.chemsrc.com/caspic/007/124750-51-2.png)
![5-[4'-(Bromomethyl)-1,1'-biphenyl-2-yl]-2-triphenylmethyl-2H-tetrazole structure](https://image.chemsrc.com/caspic/261/133051-88-4.png)
![ethyl 4-(1-hydroxy-1-methylethyl)-2-propyl-1-[[2'-[2-(triphenylmethyl)-2H-tetrazol-5-yl]biphenyl-4-yl]methyl]imidazole-5-carboxylate structure](https://image.chemsrc.com/caspic/251/172875-59-1.png)
