439239-90-4
| Name | 2,4,6-trifluoro-N-[6-(1-methylpiperidine-4-carbonyl)pyridin-2-yl]benzamide |
|---|---|
| Synonyms |
COL-144
2,4,6-trifluoro-N-[6-(1-methyl-piperidin-4-ylcarbonyl)-pyridin-2-yl]-benzamide 2,4,6-Trifluoro-N-(6-(1-methylpiperidine-4-carbonyl)pyridin-2-yl)benzamide Lasmiditan [INN] 2,4,6-trifluoro-N-{6-[(1-methylpiperidin-4-yl)carbonyl]pyridin-2-yl}benzamide 2,4,6-trifluoro-N-[6-(1-methyl-piperidin-4-ylcarbonyl)-pyridine-2-yl]-benzamide 2,4,6-Trifluoro-N-{6-[(1-methyl-4-piperidinyl)carbonyl]-2-pyridinyl}benzamide 2,4,6-trifluoro-N-(6-(1-methylpiperidine-4-carbonyl)pyridine-2-yl)benzamide Lasmiditan (USAN/INN) lasmitidan Lasmiditan Benzamide, 2,4,6-trifluoro-N-[6-[(1-methyl-4-piperidinyl)carbonyl]-2-pyridinyl]- |
| Description | Lasmiditan (COL-144; LY573144) is a high-affinity, highly selective 5-HT1F receptor agonist(Ki=2.1 nM), compared with Ki of 1043 nM and 1357 nM at the 5-HT(1B) and 5-HT(1D) receptors, respectively.IC50 value: 2.1 nM (Ki, 5-HT1F); >1000 nM (Ki, 5-HT1B/5-HT1D) [1]Target: 5-HT1F receptorin vitro: In vitro binding studies Lasmiditan showed a K(i) value of 2.21 nM at the 5-HT(1F) receptor, compared with K(i) values of 1043 nM and 1357 nM at the 5-HT(1B) and 5-HT(1D) receptors, respectively, a selectivity ratio greater than 470-fold. Lasmiditan showed higher selectivity for the 5-HT(1F) receptor relative to other 5-HT(1) receptor subtypes than the first generation 5-HT(1F) receptor agonist LY334370. Unlike the 5-HT(1B/1D) receptor agonist sumatriptan, lasmiditan did not contract rabbit saphenous vein rings, a surrogate assay for human coronary artery constriction, at concentrations up to 100 μM [1].in vivo: In two rodent models of migraine, oral administration of lasmiditan potently inhibited markers associated with electrical stimulation of the trigeminal ganglion (dural plasma protein extravasation, and induction of the immediate early gene c-Fos in the trigeminal nucleus caudalis) [1]. Two RCTs in the phase II development of lasmiditan was reviewed. In the intravenous placebo-controlled RCT, lasmiditan doses of 2.5-45 mg were used, and there was a linear association between headache relief (HR) rates and dose levels (P < 0.02). For lasmiditan 20 mg, HR was 64 % and for placebo it was 45 % (NS). In the oral placebo-controlled RCT, lasmiditan doses of 50, 100, 200 and 400 mg were used. For HR, all doses of lasmiditan were superior to placebo (P < 0.05). For lasmiditan 400 mg, HR was 64 % and it was 25 % for placebo. Adverse events (AEs) emerging from the treatment were reported by 22 % of the patients receiving placebo and by 65, 73, 87 and 87 % of patients receiving 50, 100, 200 and 400 mg, respectively [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 433.3±45.0 °C at 760 mmHg |
| Molecular Formula | C19H18F3N3O2 |
| Molecular Weight | 377.360 |
| Flash Point | 215.9±28.7 °C |
| Exact Mass | 377.135101 |
| PSA | 62.30000 |
| LogP | 1.90 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.585 |
| Storage condition | 2-8℃ |
| HS Code | 2933990090 |
|---|
|
~98% 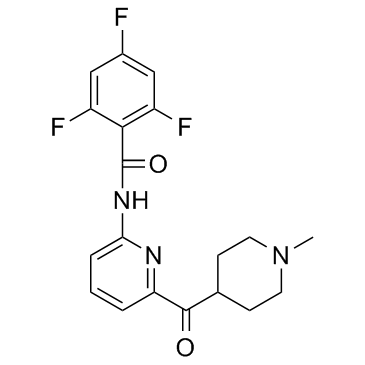
439239-90-4 |
| Literature: ELI LILLY AND COMPANY Patent: WO2003/84949 A1, 2003 ; Location in patent: Page/Page column 35 ; WO 03/084949 A1 |
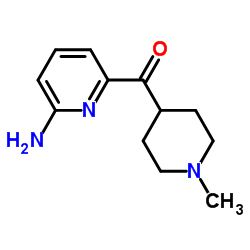
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |