212631-67-9
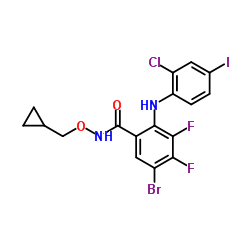
| Name | 5-bromo-2-(2-chloro-4-iodoanilino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide |
|---|---|
| Synonyms |
Benzamide, 5-bromo-2-[(2-chloro-4-iodophenyl)amino]-N-(cyclopropylmethoxy)-3,4-difluoro-
5-Bromo-2-[(2-chloro-4-iodophenyl)amino]-N-(cyclopropylmethoxy)-3,4-difluorobenzamide hms3263a07 PD 184161 |
| Description | PD184161 is an orally active MEK inhibitor. PD184161 inhibits MEK activity (IC50=10-100 nM) in a time- and concentration-dependent manner. PD184161 inhibits cell proliferation and induces apoptosis. PD184161 produces depressive-like behavior[1][2]. |
|---|---|
| Related Catalog | |
| Target |
MEK:10-100 nM (IC50) |
| In Vitro | PD184161 (1-20 μM; 24, 48, or 72 hours) inhibits cell proliferation and induces apoptosis in a time and concentration dependent manner[1]. PD184161 (0.1 and 1.0 μM; 1 hour) inhibits ERK1,2 phosphorylation[1]. PD184161 (5 μM; 30 min) prevents the toxic effects of bicuculline[3]. Cell Proliferation Assay[1] Cell Line: HCC cell lines (HepG2, Hep3B, PLC, and SKHep) Concentration: 1-20 μM Incubation Time: 24, 48, or 72 hours Result: Inhibited cell proliferation. Apoptosis Analysis[1] Cell Line: HCC cell lines (HepG2, Hep3B, PLC, and SKHep) Concentration: 1-20 μM Incubation Time: 48 hours Result: Induced cell apoptosis. Western Blot Analysis[1] Cell Line: HCC cell lines (HepG2, Hep3B, PLC, and SKHep) Concentration: 0.1 and 1.0 μM Incubation Time: 1 hours Result: Inhibited ERK1,2 phosphorylation. Cell Viability Assay[3] Cell Line: Primary mouse neurons Concentration: 5 μM Incubation Time: 30 min Result: Prevents the toxic effects of bicuculline. |
| In Vivo | PD184161 reduces tumor xenograft P-ERK levels in 3-12 hours after an oral dose[1]. PD184161 (300 mg/kg; orogastric gavage twice daily for 38 days) significantly suppresses tumor engraftment and initial growth[1]. PD184161 (30 mg/kg; i.p.; single injection) produces depressive-like behavior[2]. PD184161 (500 μg/kg; intravenous injection) prevents the progression of neurological deficits and brain damage after stroke[3]. Animal Model: Hep3B tumor xenografts BALB/c athymic nude mice[1] Dosage: 300 mg/kg Administration: Orogastric gavage twice daily for 38 days Result: Decreased the early tumor growth. Animal Model: Male, 6 weeks C57Bl/6 mice[2] Dosage: 500 μg/kg Administration: intravenously in 30 min before MCAO or PTZ administration Result: Prevented the progression of neurological deficits and brain damage after stroke. Animal Model: C57Bl/6 mice[3] Dosage: 30 mg/kg Administration: i.p., single injection Result: Produced depressive-like behavior. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Molecular Formula | C17H13BrClF2IN2O2 |
| Molecular Weight | 557.556 |
| Exact Mass | 555.886169 |
| PSA | 50.36000 |
| LogP | 9.28 |
| Index of Refraction | 1.670 |