2591-17-5
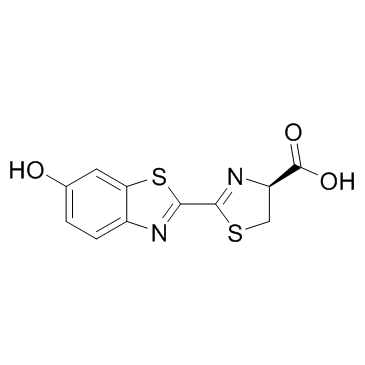
| Name | Photinus luciferin |
|---|---|
| Synonyms |
D-Leciferin
nbsp D-LUCIFERIN FIREFLY BEETLE LUCIFERIN (S)-4,5-Dihydro-2-(6-hydroxy-1,3-benzothiazol-2-yl)thiazole-4-carboxylic acid D-Luciferin (4S)-2-(6-Hydroxy-1,3-benzothiazol-2-yl)-4,5-dihydro-1,3-thiazole-4-carboxylic acid 4-Thiazolecarboxylic acid, 4,5-dihydro-2-(6-hydroxy-2-benzothiazolyl)-, (4S)- D-(-)-Luciferin LUCIFERIN EINECS 219-981-3 (S)-2-(6-Hydroxy-2-benzothiazolyl)-2-thiazoline-4-carboxylic Acid firefly liciferin Firefly luciferin MFCD00042929 (S)-4,5-Dihydro-2-(6-hydroxy-2-benzothiazolyl)-4-thiazolecarboxylicacid (S)-2-(6-Hydroxybenzo[d]thiazol-2-yl)-4,5-dihydrothiazole-4-carboxylic acid AURORA KA-6717 4-Thiazolecarboxylic acid, 4,5-dihydro-2-(6-hydroxy-2-benzothiazolyl)-, (S)- firefly luciferin IV (S)-luciferin LUCIFERIN,D |
| Description | D-luciferin is the natural substrate of luciferases that catalyze the production of light in bioluminescent insects. |
|---|---|
| Related Catalog | |
| In Vitro | D-luciferin is the natural substrate of the enzyme luciferase (Luc), that catalyzes the production of the typical yellowgreen light of fireflies.The present review covers the synthesis of D-luciferin and derivatives or analogues that are substrates or inhibitors of the luciferase from the American firefly Photinus pyralis, the enzyme more frequently used in techniques of in vitro and optical imaging[1]. |
| In Vivo | Bioluminescence imaging (BLI) using the firefly luciferase (Fluc) as a reporter gene and D-luciferin as a substrate is currently the most widely employed technique. The total signal intensity is plotted against the time after D-luciferin injection to generate a time-intensity curve. In addition to the peak signal, the signals at fixed time points (5, 10, 15, and 20 min) after D-luciferin injection are determined as alternatives to the peak signal. The signal in a given time-intensity curve is normalized for the peak signal in the curve to represent the pattern of temporal changes after D-luciferin injection[2]. |
| Animal Admin | Mice[2] In vivo BLI is performed using a cooled charge-coupled device camera system (IVIS Imaging System 100) 3, 5, 7, 10, 12, 14, 19, 21, 24, and 28 days after the inoculation of HCT116-Luc cells. Mice are injected with 75 mg/kg D-luciferin in 100 μL of phosphate-buffered saline subcutaneously near the scapula and were placed in the light-tight chamber of the imaging system under isoflurane anesthesia. Beginning 5 min after injection, dorsal luminescent images with an exposure time of 1 s are acquired sequentially at a rate of one image per min until 20 min after D-luciferin injection. Data acquisition is continued until 40 min postinjection on days 3 or 5 and until 25 min on day 7, because of the prolonged time course of light emission. Binning is 4 and the field of view is 15 cm. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 587.6±60.0 °C at 760 mmHg |
| Melting Point | 200-204ºC |
| Molecular Formula | C11H8N2O3S2 |
| Molecular Weight | 280.323 |
| Flash Point | 309.2±32.9 °C |
| Exact Mass | 279.997620 |
| PSA | 136.32000 |
| LogP | 0.87 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.865 |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36/37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
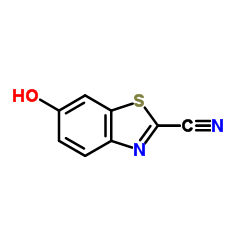
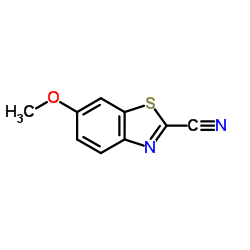
| Precursor 10 | |
|---|---|
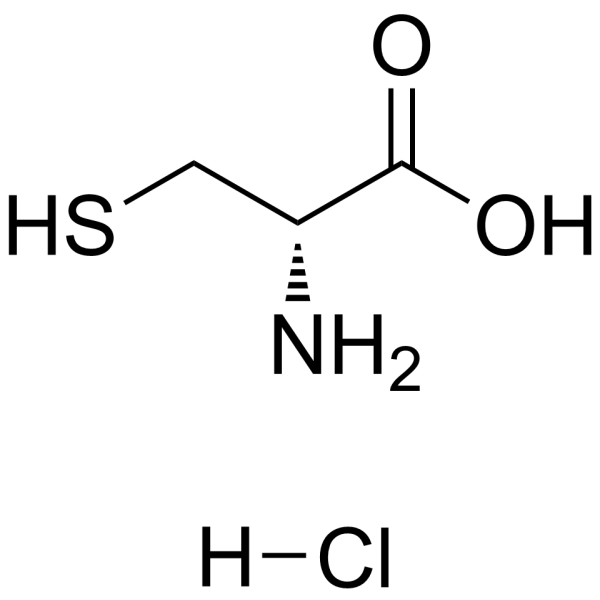
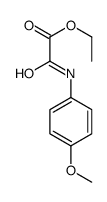
| DownStream 2 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |

![6-ETHOXYBENZO[D]THIAZOLE-2-CARBONITRILE structure](https://image.chemsrc.com/caspic/407/91634-13-8.png)
![6-METHOXYBENZO[D]THIAZOLE-2-CARBOXAMIDE structure](https://image.chemsrc.com/caspic/297/946-12-3.png)