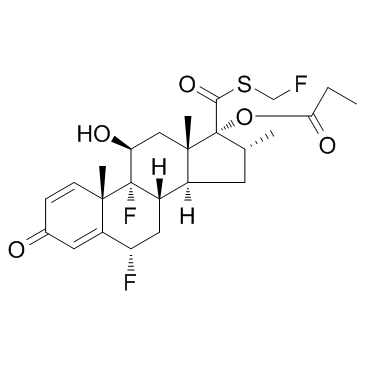
80474-14-2
| Name | fluticasone propionate |
|---|---|
| Synonyms |
Fluticasone propionate (JAN/USAN)
Flonase Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)-, S-(fluoromethyl) ester, (6α,11β,16α,17α)- FLUNASE Flovent DPI Flutide N Flixonase (6α,11β,16α,17α)-6,9-Difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl propionate Flixotide flutide MFCD08064194 Fluticasone propionate (6α,11β,16α,17α)-6,9-difluoro-17-{[(fluoromethyl)sulfanyl]carbonyl}-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl propanoate (6a,11b,16a,17a)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic Acid S-(Fluoromethyl) Ester flovent diskus Zoflut Cutivate FLOVENT Fluticasone (propionate) |
| Description | Fluticasone propionate is a high affinity, selective GR (glucocorticoid receptor) agonist which is derived from fluticasone used to treat asthma and allergic rhinitis.Target: Glucocorticoid ReceptorFluticasone propionate is a corticosteroid derived from fluticasone used to treat asthma and allergic rhinitis. It is also used to treat eosinophilic esophagitis. Fluticasone propionate is a synthetic trifluorinated glucocorticoid. It is highly lipophilic (logp octanol/water 3.69). In studies the topical drug has been associated with burning, stinging, skin irritation, blisters, dryness, skin infection, infected eczema, viral warts,impetigo, atopic dermatitis, pruritus, exacerbation of pruritus, exacerbation of eczema, erythema, and folliculitis.There are also numerous side effects associated with the oral version of this medication including headache, stuffy or runny nose, difficulty speaking, sore or irritated throat and painful white patches in the mouth or throat. Candidiasis of mouth and throat is reported as a "very common" side effect indicating that it occurs with a frequency greater than 1 in 10.Hoarseness is "common" indicating a frequency from 1 in 10 to 1 in 100. In both instances rinsing the mouth with water immediately after inhalation is recommended. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 568.3±50.0 °C at 760 mmHg |
| Melting Point | 275 °C |
| Molecular Formula | C25H31F3O5S |
| Molecular Weight | 500.571 |
| Flash Point | 297.5±30.1 °C |
| Exact Mass | 500.184418 |
| PSA | 105.97000 |
| LogP | 3.73 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Storage condition | Store at RT |
| Water Solubility | DMSO: ≥10 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | BV7980000 |
| HS Code | 2937229000 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2937229000 |
|---|