66280-25-9
| Name | Gomisin J |
|---|---|
| Synonyms |
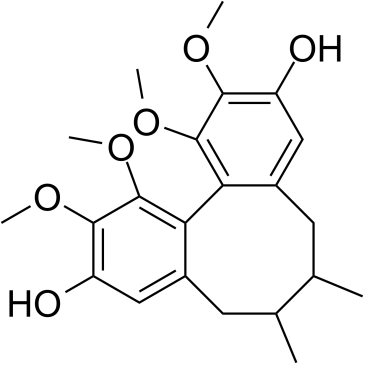
Dibenzo[a,c]cyclooctene-3,10-diol, 5,6,7,8-tetrahydro-1,2,11,12-tetramethoxy-6,7-dimethyl-, (6R,7S)-
(-)-gomisin J (6R,7S)-1,2,11,12-Tetramethoxy-6,7-dimethyl-5,6,7,8-tetrahydrodibenzo[a,c][8]annulene-3,10-diol UNII:X13A57600T 6(S),7(R)-Dibenzo(a,c)cyclooctene-3,10-diol,5,6,7,8-tetrahydro-1,2,11,12-tetramethoxy-6,7-dimethyl Dibenzo(a,c)cyclooctene-3,10-diol,5,6,7,8-tetrahydro-1,2,11,12-tetramethoxy-6,7-dimethyl-,stereoisomer |
| Description | Gomisin J is a small molecular weight lignan found in Schisandra chinensis and has been demonstrated to have vasodilatory activity[1]. Gomisin J suppresses lipid accumulation by regulating the expression of lipogenic and lipolytic enzymes and inflammatory molecules through activation of AMPK, LKB1 and Ca2+/calmodulin-dependent protein kinase II and inhibition of fetuin-A in HepG2 cells. gomisin J has potential benefits in treating nonalcoholic fatty liver disease[2]. |
|---|---|
| Related Catalog | |
| Target |
AMPK Ca2+ |
| References |
| Density | 1.161 |
|---|---|
| Boiling Point | 587.5±50.0 °C at 760 mmHg |
| Melting Point | 148-149 ºC |
| Molecular Formula | C22H28O6 |
| Molecular Weight | 388.454 |
| Flash Point | 309.1±30.1 °C |
| Exact Mass | 388.188599 |
| PSA | 77.38000 |
| LogP | 4.80 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.556 |
| Hazard Codes | Xi |
|---|
| Precursor 2 | |
|---|---|
| DownStream 0 | |