28380-24-7
| Name | nigericin |
|---|---|
| Synonyms |
Nigericin
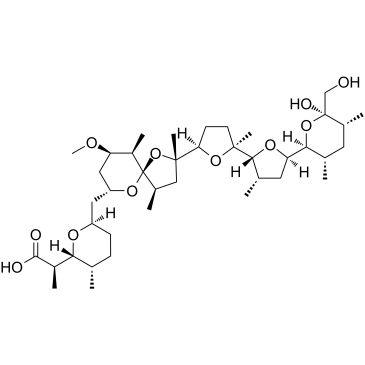
HELIXIN C 2H-Pyran-2-acetic acid, tetrahydro-6-[[(2S,4R,5R,9R,10R)-9-methoxy-2,4,10-trimethyl-2-[(2S,3'R)-octahydro-2,3'-dimethyl-5'-[(3S,5R,6R)-tetrahydro-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyl-2H-pyran-2-yl][2,2'-bifuran]-5-yl]-1,6-dioxaspiro[4.5]dec-7-yl]methyl]-α,3-dimethyl-, sodium salt, (αS,3R)- (1:1) Sodium (2S)-2-[(3R)-6-{[(2S,4R,5R,9R,10R)-2-{(2S,3'R)-5'-[(3S,5R,6R)-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyltetrahydro-2H-pyran-2-yl]-2,3'-dimethyloctahydro-2,2'-bifuran-5-yl}-9-methoxy-2,4,10-trimethyl-1,6-dioxaspiro[4.5]dec-7-yl]methyl}-3-methyltetrahydro-2H-pyran-2-yl]propanoate ANTIBIOTIC K178 NIGERICIN NA AZALOMYCIN M 2H-Pyran-2-acetic acid, tetrahydro-6-[[(2S,4R,5R,9R,10R)-9-methoxy-2,4,10-trimethyl-2-[(2S,3'R)-octahydro-2,3'-dimethyl-5'-[(3S,5R,6R)-tetrahydro-6-hydroxy-6-(hydroxymethyl)-3,5-dimethyl-2H-pyran-2-yl ;][2,2'-bifuran]-5-yl]-1,6-dioxaspiro[4.5]dec-7-yl]methyl]-α,3-dimethyl-, sodium salt, (αS,3R)- (1:1) POLYETHERIN A Helix c x-464 |
| Description | Nigericin is an antibiotic derived from Streptomyces hygroscopicus that act as a K+/H+ ionophore, promoting K+/H+ exchange across mitochondrial membranes[1].Nigericin can be a NLRP3 activator that induces the release of IL-1β as a NALP3-dependent manner[2]. Nigericin triggers eryptosis, an effect paralleled by ROS formation, and in part due to induction of oxidative stress. Nigericin triggers apoptosis[3]. |
|---|---|
| Related Catalog | |
| Target |
K+/H+ ionophore[1] |
| References |
| Density | 1.19g/cm3 |
|---|---|
| Boiling Point | 779.9ºC at 760mmHg |
| Melting Point | 245-255ºC |
| Molecular Formula | C40H68O11 |
| Molecular Weight | 746.943 |
| Flash Point | 226.9ºC |
| Exact Mass | 746.458130 |
| PSA | 145.20000 |
| LogP | 4.37090 |
| Index of Refraction | 1.543 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T: Toxic; |
|---|---|
| RIDADR | UN 3462 6 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| Precursor 0 | |
|---|---|
| DownStream 1 | |