58970-76-6
| Name | Ubenimex |
|---|---|
| Synonyms |
BESTATIN
BENIMEX Bestatin,UbeniMex 2-(3-Amino-2-hydroxy-4-phenyl-butyrylamino)-4-methyl-pentanoic acid nk421 MFCD00083262 N-(3-Amino-2-hydroxy-4-phenylbutanoyl)leucine EINECS 261-529-2 |
| Description | Bestatin is a natural, broad-spectrum, and competitive aminopeptidase inhibitor. |
|---|---|
| Related Catalog | |
| In Vitro | Bestatin enhances ATRA-induced differentiation and inhibits ATRA-driven phosphorylation of p38 MAPK in ATRA-sensitive APL NB4 cells. Bestatin can not reverse the differentiation block in ATRA-resistant APL MR2 cells. CD13 ligation with anti-CD13 antibody WM-15 results in phosphorylation of p38 MAPK, reduces the inhibition of Bestatin on the phosphorylation of p38 MAPK, and completely abolishes the enhancement of Bestatin on ATRA-inducing differentiation in NB4 cells[2]. Bestatin (600 μM)-treated cells progress slower through the cell cycle due to decreased rate of cell growth and the frequency of cell division. Bestatin inhibits the frequency of mitosis and the inherent multinuclearity in D. discoideum, and is not cytotoxic to D. discoideum cells at 0-600 μM. Bestatin inhibits aminopeptidase activity in lysates of PsaA-GFP- and GFP-expressing cells by 69.39% ± 10.5% and 39.93% ± 18.7% of control, respectively[4]. |
| In Vivo | Bestatin (20 μM) significantly reduces CD13 expression in diabetic mice and results a significant inhibition of MMP-9 specific gelationolytic band densities compared to diabetic vehicle-treated mice. Bestatin treatment significantly inhibits the expression of VEGF and heparanase in diabetic mice. Intravitreal bestatin treatment significantly downregulates the expression of both HIF-1α and VEGF in diabetic mice retinas. Furthermore, the upregulated expression of heparanase in diabetic mice retinas is significantly inhibited by intravitreal bestatin treatment[1]. Bestatin (10, 1, and 0.1mg/kg, i.p.) treatment before the antigen-potentiated humoral response to SRBC results in an increased number of splenocytes producing hemolytic anti-SRBC antibodies (PFC) and the 2-ME-resistant serum hemagglutinin titer (at a dose of 0.1 mg/kg). Bestatin (1 and 0.1 mg/kg) administered to mice five times on alternate days after cyclophosphamide injection does not change the suppressive effect of the drug regarding the number of PFC, and even causes the further decrease of the total anti-SRBC hemagglutinins at dose of 1 mg/kg on day 7 after antigen stimulation[3]. |
| Kinase Assay | Cells are harvested, washed, and lysed in NP-40 lysis buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.5% NP-40). Total cell protein is quantified using the Bradford assay and 1-mg/mL protein aliquots are made. Ten microliters of total cell protein is mixed with 290 μL of substrate solution (0.1 mg/mL dithiothreitol [DTT], 0.1 mg/mL albumin, and 1 mM alanine-β-naphthylamide). Fluorometric measurements (340 nm excitation, 400 nm emission) are made after 15 and 30 min. The slope of the line between the 15- and 30-min measurements is used to represent aminopeptidase activity. Total cell protein is preincubated with bestatin, amastatin, puromycin, EDTA, and/or ZnCl2 for 20 min before the fluorometric aminopeptidase assay. |
| Cell Assay | Growing cells (1×106 to 2×106 cells/mL) are diluted to 1.0×103 cells/mL and transferred (3 mL) into a well in a 12-well multiwell plate (2.5-cm diameter/well). Cells are treated with 0, 10, 50, 100, 300, or 600 μM Bestatin and allowed to grow at 21°C shaking at 180 rpm for 48 h. A hemocytometer is used to measure cell density after 0, 24, and 48 h. |
| Animal Admin | Bestatin is dissolved in PBS. The agent (doses of 10, 1, and 0.1 mg/kg) is injected i.p. to non-cyclophosphamide-treated mice, 5 or 10 times at 24-h intervals before SRBC immunization. The mice are immunized 24 h after the last dose of bestatin. Pharmacological immunosuppression is induced by a single intraperitoneal injection of cyclophosphamide administered at a dose of 350 mg/kg, 12 days before SRBC immunization. Bestatin at the doses of 1 and 0.1 mg/kg is injected to cyclophosphamide-immunosuppressed mice i.p. five times at 48-h intervals or 10 times at 24-h intervals before SRBC immunization. The first dose of bestatin is administered 24 h after cyclophosphamide, while the last dose of the drug is injected 24h before SRBC immunization. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 604.7±55.0 °C at 760 mmHg |
| Melting Point | 245 °C (dec.)(lit.) |
| Molecular Formula | C16H24N2O4 |
| Molecular Weight | 308.373 |
| Flash Point | 319.5±31.5 °C |
| Exact Mass | 308.173615 |
| PSA | 112.65000 |
| LogP | 2.64 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.557 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | OH2915000 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
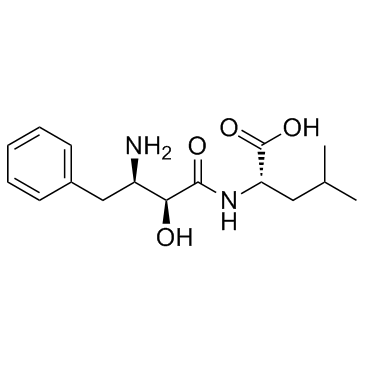
![N-[(2S,3S)-3-azido-2-hydroxy-4-phenylbutanoyl]-L-leucine benzyl ester structure](https://image.chemsrc.com/caspic/387/121445-53-2.png)
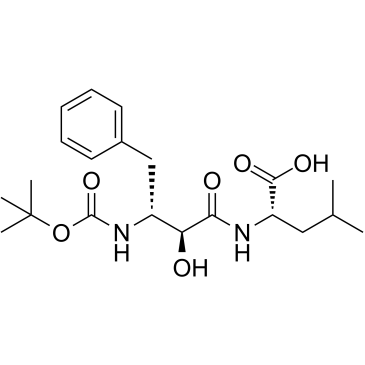
![benzyl N-[(2S,3R)-3-(N-benzyloxycarbonyl)amino-2-hydroxy-4-phenylbutanoyl]-L-leucinate structure](https://image.chemsrc.com/caspic/035/60016-66-2.png)
![methyl N-{(2S,3R)-2-O-benzyl-4-phenyl-3-[9-phenyl-9-fluorenyl-amino]-butanoyl}-L-leucine structure](https://image.chemsrc.com/caspic/109/577979-03-4.png)
![Benzyl [(2R)-1-oxo-3-phenyl-2-propanyl]carbamate structure](https://image.chemsrc.com/caspic/297/63219-70-5.png)